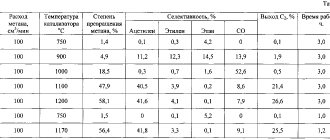
Chemical properties
Reactions in aqueous solutions
Copper(I) oxide does not react with water. To a very small extent (PR = 1.2⋅10−15) dissociates:
Cu2O + H2O ⇄ 2Cu+ + 2OH−
Disproportionation equilibrium:
2Cu+ ⇄ Cu2+ + Cu
Copper (I) oxide is transferred into solution:
- concentrated hydrochloric acid
Cu2O + 4HCl ⟶ 2H[CuCl2] + H2O
- concentrated alkali (partially)
Cu2O + 2OH− + H2O ⇄ 2[Cu(OH)2]−
- concentrated ammonia hydrate and concentrated solutions of ammonium salts
Cu2O + 4(NH3 ⋅ H2O) ⟶ 2[Cu(NH3)2]OH + 3H2O Cu2O + 2NH4+ ⟶ 2[Cu(H2O)(NH3)]+
- by oxidation to copper (II) salts with various oxidizing agents (for example, concentrated nitric and sulfuric acids, oxygen in dilute hydrochloric acid)
Cu2O + 6HNO3 ⟶ 2Cu(NO3)2 + 2NO2↑ + 3H2O Cu2O + 3H2SO4 ⟶ 2CuSO4 + SO2↑ + 3H2O 2Cu2O + 8HCl + O2 ⟶ 4CuCl2 + 4H2O
Also, copper (I) oxide enters into the following reactions in aqueous solutions:
- slowly oxidized by oxygen to copper(II) hydroxide
2Cu2O + 4H2O + O2 ⟶ 4Cu(OH)2↓
- reacts with dilute hydrohalic acids to form the corresponding copper(I) halides:
Cu2O + 2HHal ⟶ 2CuHal↓ + H2O (Hal = Cl, Br, I)
- in dilute sulfuric acid dismutates into copper (II) sulfate and metallic copper
Cu2O + H2SO4 ⟶ CuSO4 + Cu↓ + H2O
- reduced to copper metal by typical reducing agents, e.g. sodium hydrogen sulfite in concentrated solution
2Cu2O + 2NaHSO3 ⟶ 4Cu↓ + Na2SO4 + H2SO4
Reactions at high temperatures
Copper(I) oxide is reduced to copper metal in the following reactions:
- when heated to 1800 °C (decomposition)
2Cu2O →1800∘C 4Cu + O2
- when heated in a stream of hydrogen, carbon monoxide, with aluminum
Cu2O + H2 →>250∘C 2Cu + H2O Cu2O + CO →250−300∘C 2Cu + CO2 3Cu2O + 2Al →1000∘C 6Cu + Al2O3
- when heated with sulfur
2Cu2O + 3S →>600∘C 2Cu2S + SO2 2Cu2O + Cu2S →1200−1300∘C 6Cu + SO2
Copper (I) oxide can be oxidized to copper (II) compounds in a stream of oxygen or chlorine:
2Cu2O + O2 →500∘C 4CuO Cu2O + Cl2 →250∘C Cu2Cl2O
Also, at high temperatures, copper (I) oxide reacts:
- with ammonia (copper(I) nitride is formed)
3Cu2O + 2NH3 →250∘C 2Cu3N + 3H2O
- with oxides of alkali metals and barium (double oxides are formed)
Cu2O + M2O →600−800∘C 2MCuO Cu2O + BaO →500−600∘C BaCu2O2
Other reactions
Copper(I) oxide reacts with hydrogen azide:
- upon cooling, a precipitate of copper (II) azide precipitates
Cu2O + 5HN3 →10−15∘C 2Cu(N3)2↓ + H2O + NH3↑ + N2↑
- at room temperature in a stream of hydroazidic acid a precipitate of copper (I) azide precipitates
Cu2O + 2HN3 →20−25∘C 2CuN3↓ + H2O
Copper oxide
Cuprum (Cu) is one of the low-active metals. It is characterized by the formation of chemical compounds with oxidation states +1 and +2. So, for example, two oxides, which are a compound of two elements Cu and oxygen O: with an oxidation state of +1 - copper oxide Cu2O and an oxidation state of +2 - copper oxide CuO. Despite the fact that they consist of the same chemical elements, each of them has its own special characteristics. In the cold, the metal interacts very weakly with air oxygen, becoming covered with a film of copper oxide, which prevents further oxidation of cuprum. When heated, this simple substance with serial number 29 in the periodic table is completely oxidized. In this case, copper (II) oxide is also formed: 2Cu + O2 → 2CuO.
Nitrous oxide is a brownish-red solid with a molar mass of 143.1 g/mol. The compound has a melting point of 1235°C and a boiling point of 1800°C. It is insoluble in water, but soluble in acids. Copper oxide (I) is diluted in ammonia solution (concentrated), resulting in the formation of a colorless complex +, which is easily oxidized in air to a blue-violet ammonia complex 2+, dissolving in hydrochloric acid to form CuCl2. In the history of semiconductor physics, Cu2O is one of the most studied materials.
Copper(I) oxide, also known as hemioxide, has basic properties. It can be obtained by oxidation of the metal: 4Cu + O2 → 2 Cu2O. Impurities such as water and acids affect the rate of this process, as well as further oxidation to divalent oxide. Cuprous oxide can dissolve in sulfuric acid, resulting in the formation of pure metal and salt: H2SO4 + Cu2O → Cu + CuSO4 + H2O. According to a similar scheme, the interaction of an oxide with the oxidation state of the metal +1 with other oxygen-containing acids occurs. When hemioxide reacts with halogen-containing acids, monovalent metal salts are formed: 2HCl + Cu2O → 2CuCl + H2O.
Copper(I) oxide occurs naturally in the form of red ore (an obsolete name, along with ruby Cu), called the mineral "Cuprite". It takes a long time to form. It can be produced artificially at high temperatures or under high oxygen pressure. Hemioxide is commonly used as a fungicide, as a pigment, as an antifouling agent in underwater or marine paint, and is also used as a catalyst.
However, the effects of this substance with the chemical formula Cu2O on the body can be dangerous. If inhaled, causes shortness of breath, cough, and ulceration and perforation of the respiratory tract. If ingested, it irritates the gastrointestinal tract, which is accompanied by vomiting, pain and diarrhea.
Higher copper oxide is a brown to black powder in appearance. In nature, it is found in its pure form as the mineral “Tenorite”. Its melting point is 1326°C, boiling point is 2000°C. It is insoluble in water, alcohol, ammonium hydroxide, ammonium carbonate solution. Soluble in aqueous solutions of ammonium chloride and potassium cyanide. This black solid can be produced by heating Cu in air. However, in this case, Cu oxide is also formed. The production of copper oxide CuO is possible by heating the following compounds:
- copper (II) nitrate 2Cu(NO3)2 → 4 NO2+ O2 + 2CuO;
- copper (II) hydroxide Cu(OH)2 → H2O + CuO;
- copper (II) carbonate CuCO3 → CO2 + CuO.
Cuprum(II) oxide is basic, so it dissolves in mineral acids (hydrochloric, sulfuric and nitric) to give the corresponding divalent Cu salt:
- 2HCl + CuO → CuCl2 + H2O;
- H2SO4 + CuO → CuSO4 + H2O;
- 2HNO3 + CuO → Cu(NO3)2 + H2O.
Copper (II) oxide reacts with concentrated alkali to form a salt: 2 KOH + CuO + H2O → K2.
The oxide can also be reduced to metallic Cu by reaction with hydrogen or carbon monoxide:
- H2 + CuO → Cu + H2O;
- CO + CuO → Cu + CO2.
Copper(II) oxide is used in ceramics (as a pigment) to produce glazes (blue, green and red, and sometimes pink, gray or black). It is also used as a dietary supplement in animals to reduce cuprum deficiency in the body. This is an abrasive material that is necessary for polishing optical equipment. It is used for the production of dry batteries, to obtain other Cu salts. The CuO compound is also used in welding copper alloys.
Exposure to the chemical compound CuO can also be dangerous to the human body. Causes lung irritation if inhaled. Copper(II) oxide can cause metal fume fever (MFF). Cu oxide causes skin discoloration and vision problems may occur. If it enters the body, like hemioxide, it leads to poisoning, which is accompanied by symptoms in the form of vomiting and pain.
Receipt
Copper(I) oxide can be prepared:
- heating copper metal in the absence of oxygen
4Cu + O2 →>200∘C 2Cu2O
- heating copper metal in a stream of nitric oxide (I) or nitric oxide (II)
2Cu + N2O →500−600∘C Cu2O + N2 4Cu + 2NO →500−600∘C 2Cu2O + N2
- heating copper metal with copper(II) oxide
Cu + CuO →1000−1200∘C Cu2O
- thermal decomposition of copper (II) oxide
4CuO →1026−1100∘C 2Cu2O + O2
- heating copper(I) sulfide in a stream of oxygen
2Cu2S + 3O2 →1200−1300∘C 2Cu2O + 2SO2
In laboratory conditions, copper (I) oxide can be obtained by reducing copper (II) hydroxide (for example, with hydrazine):
4Cu(OH)2 + N2H4 ⋅ H2O →100∘C 2Cu2O ↓ + N2↑ + 7H2O
Also, copper(I) oxide is formed in ion exchange reactions of copper(I) salts with alkalis, for example:
- in the reaction of copper (I) iodide with a hot concentrated solution of potassium hydroxide
2CuI + 2KOH ⟶ Cu2O↓ + 2KI + H2O
- in the reaction of hydrogen dichlorocuprate (I) with a dilute solution of sodium hydroxide
2H[CuCl2] + 4NaOH ⟶ Cu2O↓ + 4NaCl + 3H2O
In the last two reactions, a compound with a composition corresponding to the formula CuOH (copper (I) hydroxide) is not formed. The formation of copper (I) oxide occurs through an intermediate hydrate form of variable composition Cu2O ⋅ xH2O.
- Oxidation of aldehydes with copper (II) hydroxide. If an aldehyde solution is added to the blue precipitate of copper (II) hydroxide and the mixture is heated, then a yellow precipitate of copper (I) hydroxide appears first:
R−CHO + 2Cu(OH)2 →t R−COOH + 2CuOH↓ + H2O upon further heating, the yellow precipitate of copper (I) hydroxide turns into red copper (I) oxide: 2CuOH →tCu2O + H2O
Brief characteristics of copper (II) oxide:
Copper (II) oxide is a black inorganic substance.
Since the valence of copper varies and is equal to one, two or three, copper oxide contains, respectively, two copper atoms and one oxygen atom, one copper atom and one oxygen atom, two copper atoms and three oxygen atoms.
Cuprous oxide contains one copper atom and one oxygen atom, respectively.
The chemical formula of copper (II) oxide is CuO.
Powder. Does not dissolve in water.
Chemical properties of copper (II) oxide. Chemical reactions of copper (II) oxide:
Copper (II) oxide is a basic oxide.
The chemical properties of copper(II) oxide are similar to those of basic oxides of other metals. Therefore, it is characterized by the following chemical reactions:
1. reaction of copper (II) with hydrogen:
CuO + H2 → Cu + H2O (t = 300 oC).
The reaction produces copper and water.
2. reaction of copper (II) oxide with carbon:
CuO + C → Cu + CO (t = 1200 oC).
The reaction produces copper and carbon monoxide.
3. reaction of copper (II) with sulfur:
CuO + 2S → Cu + S2O (t = 150-200 oC).
The reaction takes place in a vacuum. As a result of the reaction, copper and sulfur oxide are formed.
4. reaction of copper (II) with aluminum:
3CuO + 2Al → 3Cu + Al2O3 (t = 1000-1100 oC).
As a result of the reaction, copper and aluminum oxide are formed.
5. reaction of copper (II) with copper:
CuO + Cu → Cu2O (t = 1000-1200 oC).
As a result of the reaction, copper (I) oxide is formed.
6. reaction of copper (II) with lithium oxide:
CuO + Li2O → Li2CuO2 (t = 800-1000 oC, O2).
The reaction takes place in a flow of oxygen. As a result of the reaction, lithium cuprate is formed.
7. reaction of copper (II) with sodium oxide:
CuO + Na2O → Na2CuO2 (t = 800-1000 oC, O2).
The reaction takes place in a flow of oxygen. As a result of the reaction, sodium cuprate is formed.
8. reaction of copper (II) with carbon monoxide:
CuO + CO → Cu + CO2.
The reaction produces copper and carbon monoxide (carbon dioxide).
9. reaction of copper (II) with iron oxide:
CuO + Fe2O3 → CuFe2O4 (to).
As a result of the reaction, a salt is formed - copper ferrite. The reaction occurs when the reaction mixture is calcined.
10. reaction of copper (II) with hydrofluoric acid:
CuO + 2HF → CuF2 + H2O.
As a result of a chemical reaction, a salt is obtained - copper fluoride and water.
11. reaction of copper (II) with nitric acid:
CuO + 2HNO3 → 2Cu(NO3)2 + H2O.
As a result of a chemical reaction, salt is obtained - copper nitrate and water.
(II) with other acids proceed similarly
12. reaction of copper (II) with hydrogen bromide (hydrogen bromide):
CuO + 2HBr → CuBr2 + H2O.
As a result of a chemical reaction, salt is obtained - copper bromide and water.
13. reaction of copper (II) with hydrogen iodide:
CuO + 2HI → CuI2 + H2O.
As a result of a chemical reaction, salt is obtained - copper iodide and water.
14. reaction of copper (II) with sodium hydroxide:
CuO + 2NaOH → Na2CuO2 + H2O.
As a result of a chemical reaction, salt is obtained - sodium cuprate and water.
15. reaction of copper (II) with potassium hydroxide:
CuO + 2KOH → K2CuO2 + H2O.
As a result of a chemical reaction, salt is obtained - potassium cuprate and water.
16. reaction of copper (II) with sodium hydroxide and water:
CuO + 2NaOH + H2O → Na2[Cu2(OH)]2 (t = 100 oC).
Sodium hydroxide is dissolved in water. A solution of sodium hydroxide in water 20-30%. The reaction occurs at boiling point. As a result of a chemical reaction, sodium tetrahydroxycuprate is obtained.
17. reaction of copper (II) with potassium superoxide:
2CuO + 2KO2 → 2KCuO2 + O2 (t = 400-500 oC).
As a result of a chemical reaction, a salt is obtained - potassium cuprate (III) and oxygen.
18. reaction of copper (II) with potassium peroxide:
2CuO + 2K2O2 → 2KCuO2 (t = 700 oC).
As a result of a chemical reaction, a salt is obtained - potassium cuprate (III).
19. reaction of copper (II) with sodium peroxide:
2CuO + 2Na2O2 → 2NaCuO2 (t = 700 oC).
As a result of a chemical reaction, a salt is obtained - sodium cuprate (III).
20. reaction of copper (II) with ammonia:
3CuO + 2NH3 → N2 + 3Cu + 3H2O (t = 500-550 oC).
Ammonia is passed through heated copper(II) oxide. The chemical reaction produces nitrogen, copper and water.
6CuO + 4NH3 → 2Cu3N + N2 + 6H2O (t = 250-300 oC).
As a result of a chemical reaction, copper nitride, nitrogen and water are obtained.
21. reaction of copper (II) oxide and aluminum iodide:
6CuO + 4AlI3 → 6CuI + 2Al2O3 + 3I2 (t = 230 oC).
As a result of a chemical reaction, salt is obtained - copper iodide, aluminum oxide and iodine.