Graphite
- a mineral from the class of native elements, one of the allotropic modifications of carbon. A common mineral in nature. It is usually found in the form of individual flakes, plates and clusters, varying in size and graphite content. There are deposits of crystalline graphite associated with igneous rocks or crystalline shales, and cryptocrystalline graphite formed during the metamorphism of coals.
- Structure
- Properties
- Morphology
- Origin
- Application
- Classification
- Physical properties
- Optical properties
- Crystallographic properties
See also:
Agate
- price and medicinal, magical properties
Physical properties and structure of diamond
What is graphite
Everyone knows what graphite looks like. This is the core lead of an ordinary pencil:
- The mineral is soft, which is easy to verify: pencils break due to careless handling.
- It is greasy to the touch and has different hardness and density, as indicated by the brands of pencil - from soft to hard.
- Colors and shades - a full gray range with a matte or metallic sheen.
The layered structure of the mineral creates the ability to write or draw.
Graphite is a mineral, a natural crystalline modification of carbon. Closest relatives are diamond, lonsdaleite, charoite. They are distinguished by their structure. In graphite it is layered.
Graphite can be turned into diamond by heating it to 2000°C and placing it under pressure of hundreds of atmospheres.
In nature, “pure” minerals are not found; rare, valuable metals are found among the impurities.
The production of artificial graphite has been established.
STRUCTURE
Hexagonal crystalline polymorphic (allotropic) modification of pure carbon, the most stable under the conditions of the earth's crust. The layers of the crystal lattice can be differently positioned relative to each other, forming a whole range of polytypes, with symmetry from hexagonal system (dihexagonal-dipyramidal type of symmetry) to trigonal (ditrigonal-scalenohedral symmetry). The crystal lattice of graphite is layered. In layers, C atoms are located at the sites of hexagonal cells of the layer. Each C atom is surrounded by three neighboring ones with a distance of 1.42Α
There are two modifications of graphite: α-graphite (hexagonal P63/mmc) and β-graphite (rhombohedral R(-3)m). They differ in the packing of layers. In α-graphite, half of the atoms of each layer are located above and below the centers of the hexagon (laying...AVAVAVA...), and in β-graphite, every fourth layer repeats the first. It is convenient to represent rhombohedral graphite along hexagonal axes to show its layered structure.
β-graphite is not observed in its pure form, since it is a metastable phase. However, in natural graphites the content of the rhombohedral phase can reach 30%. At a temperature of 2500-3300 K, rhombohedral graphite completely transforms into hexagonal graphite.
Story
The history and time of formation of graphite remains a mystery to science: it is too similar to other minerals in description.
The only clue is clay utensils from the Boyan Maritsa culture (the territory of modern Bulgaria and Romania, 6 thousand years ago). The products are painted with graphite paints.
Abraham Werner suggested calling the mineral graphite. This famous chemist, who “baptized” dozens of stones, took as a basis the property of the mineral to leave a clear trace of color.
The ancient Greek term γράφω means “I write.”
On the territory of Russia, graphite was found in 1826 in the Urals.
In history and literature, the mineral also appears as black/silver lead and iron carbide.
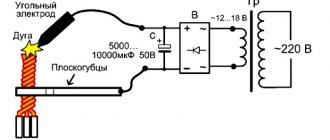
Does pencil lead raise temperature?
As one school legend goes, the core of this drawing tool is a surefire way to trick unsuspecting parents.
The "canonical" way of using it is as follows:
- Peel the wooden shell of the pencil so that only the core remains;
- You can purchase a set of spare compass leads;
- Grind the hard pieces until a black powder forms;
- Take the medicine internally;
- Drink plenty of clean boiled water;
- The result will come in half an hour.
The effectiveness of this composition is allegedly
magnificent - the mercury column confidently occupies a position from 37.5 to 38 degrees. The effect is quite short-lived, lasting a few hours. But you don’t need more to convince parents that you don’t have to go to school.
Currently, synthetic materials are increasingly used
. Therefore, testing the realism of the old Soviet tale for yourself is quite problematic, even if you want to.
Physico-chemical characteristics
According to chemical nomenclature, the mineral graphite is pure carbon with a formula of one symbol (C).
The composition is sometimes supplemented by absorbed gas, bitumen, water, and mechanical impurities.
| Formula | C (carbon) |
| Color | Grey, black steel |
| Stroke color | Black |
| Shine | Metallic |
| Transparency | Opaque |
| Hardness | 1–2 |
| Cleavage | Perfect by {0001} |
| Density | 2.09–2.23 g/cm³ |
| singonia | Hexagonal (planaxial) |
The mineral class according to international nomenclature is native element. According to the taxonomy of the USSR, it is a non-metal, but is endowed with characteristics inherent in metals - electrical conductivity, magnetism.
Basic properties
Graphite is a gray substance with a metallic luster. It has high thermal conductivity (3.55 W/degree/cm). Due to this, graphite is actively used in various fields of industry. This figure is higher than that of brick, which is explained by the presence of mobile electrons in the crystal lattice. They also promote good electrical conductivity. In all states of aggregation, this substance is characterized by low current resistance (from 0.4 to 0.6 Ohm).
Graphite is an inert substance that is not dissolved by chemically active components. This is only possible when it enters a molten metal environment with a high boiling point. Under such conditions, graphite melts completely, forming carbides.
Low coefficient of friction and high melting point provide good sealing properties. The density of graphite (kg/m3) is 2.23. But at the same time, the material bends and cuts well.
Where and how is it mined?
There are commercial-scale graphite deposits on all continents:
- Both Americas - USA, Canada, Brazil;
- Europe – Germany, Greenland, Italy;
- Australia.
The raw materials of each graphite mine can be distinguished by structure, color, and other characteristics.
Russia has three largest deposits:
- Buryatia – high-quality densely crystalline raw materials.
- Krasnodar region (two) – dense, finely crystalline, scaly, graphite shales.
Graphites are formed by coal pyrolysis or under the influence of extremely high temperatures and pressure. For example, the outpouring of magma onto coal deposits.
It is mined by above-ground or underground methods. Graphite crystals are found in shales, marbles, and other organic rocks.
The annual global production of graphite is 600 thousand tons.
Description of graphite. Properties of graphite. Application of graphite
Description and properties of graphite
Graphite is a natural element, an easily split mineral, one of the modifications of carbon. Graphite is a very soft material, easy to machine, and has a metallic luster. Graphite formula is C (carbon).
The electrical conductivity of graphite, the photo of which can be seen above, exceeds mercury electrical conductivity by 2.5 times. The specific resistance of electric current with a temperature of 0 degrees is in the range of 0.390-0.602 Ohms, and its lowest value for different types of this element is the same - 0.0075 Ohms.
The element is characterized by increased thermal conductivity, the coefficient of which is 5 times higher than that of brick (0.041). Graphite copper chains have lower thermal conductivity. The melting temperature limits are 3845-3890 C, boiling begins at 4200 C. During the combustion of the element, 7832 kcal of heat is released. Graphite is diamagnetic.
Its main chemical properties are inertness towards liquids, gases and solids, the ability to dissolve in molten metals with a melting point higher than its own. At high temperatures it can interact with other elements.
It is not elastic, but at the same time bends and cuts. Due to its fat content and plasticity, it is widely used in industrial production. Its fat content also allows it to be used as a lubricant. Graphite density 2.23 g/cm3.
Graphite has a layered structure that has its own characteristics. The carbon atoms of the graphite crystal lattice are honeycomb cells: hexagons arranged in rows. In each row, the atoms are tightly connected to each other, and the rows have a weak connection with each other. Therefore, graphite is easy to break even if you press lightly.
Hardness on the Mohs scale is equal to one, while that of diamond is ten, despite the fact that diamond and graphite are carbon subspecies. It's all about the crystal lattice. In diamond, one carbon atom is bonded to four adjacent ones. Based on research, scientists have proven that the graphite crystal lattice at temperatures above 1500 C can transform into a diamond lattice.
During processing, both the physical and chemical properties of graphite change, so it has been classified into grades that have corresponding differences. In industry, a separate grade of graphite is used for a specific type of product.
Graphite is divided into natural (natural) and artificial. During its production, properties are taken into account depending on the purpose of the product. Natural graphite, in turn, is divided into crystalline and cryptocrystalline graphite , which is a powder similar to gunpowder.
Manufacturers of graphite products set their requirements for raw materials depending on their purpose. In accordance with this, marking has been carried out, and now various grades of graphite , each with its own purpose.
Among them are electric coal, foundry, cell, battery, pencil, lubricant, as well as a special grade for the production of graphite for nuclear reactors. All graphite produced must meet technical requirements depending on its application.
Graphite deposits and mining
Graphite resources worldwide are approximately 600 million tons, and its annual production is over 600 thousand tons. The largest reserves are held by Mexico, China, the Czech Republic, Brazil, Ukraine, Russia, South Korea, and Canada. This mineral was formed by the metamorphosis of sedimentary rocks from organic compounds. Graphite deposits have been of interest from an economic point of view since ancient times and are estimated to have a capacity of millions of tons.
The development of these deposits provides the industry with the necessary raw materials. Natural graphite is found in the form of dense crystalline or fibrous inclusions in granites, calcareous rocks, gneisses, and mica. It forms large clumps as opaque, grey, sallow or scaly masses. The color of graphite ranges from steel gray to black. Lump graphite is mined underground, and graphite ore is mined by open pit mining.
Application of graphite
Both manufacturers and ordinary people have long been familiar with graphite substance , which has proven itself to have qualities that allow it to be used not only for production processes, but also in everyday life.
Due to such basic properties as electrical conductivity and fire resistance, this mineral has found wide application in industry. Metallurgy uses it for the manufacture of refractory ladles, molds for alloys, and containers for crystallization. Foundries use graphite powder as a lubricant for casting molds.
It is one of the components in the manufacture of refractory bricks. Polishing and grinding pastes are obtained from graphite mixtures. Considering the electrically conductive properties of the natural element, it is indispensable for the manufacture of contacts for electrical devices and electrodes.
The industry for the production of graphite pencils, lubricants and paints also cannot do without this substance. Pencil leads are made from black graphite gray graphite with a steely sheen exists in nature It is a plastic filler, with its help the production of artificial diamonds has been established.
Even the nuclear power industry appreciated the properties of graphite and began using it. Mechanical engineering – material for bearings, sealing and piston rings. In everyday life, they also began to use graphite lubricant - to process car springs, bicycle chains, even door hinges.
A paint product with anti-corrosion properties is graphite paint . It is a one-component suspension. In addition to the graphite filler, its composition includes a plasticizer and binding pigments. Using this paint, concrete, steel, wood, aluminum, and cast iron products are protected from corrosion.
In medicine, graphite has established itself as one of the homeopathic remedies for skin diseases resulting from internal disorders that are difficult to treat. Prevents the formation of adhesions and scars after inflammation, and also affects metabolic processes. It is difficult to list the diseases that graphite has a beneficial effect on, so it is included in many medications.
Graphite price
The sale of graphite is carried out by specialized companies engaged in the extraction and processing of graphite, the prices of which are quite reasonable. The price category of natural graphite depends on the size of its crystals and carbon content. Each grade of graphite has its own cost - the higher the carbon content, the better the technical properties, and the more expensive it is.
This mineral is sold both retail and wholesale. The consumer can buy graphite on favorable terms. When purchasing in bulk, a discount is given and delivery is ensured. The cost also depends on the region. The average price for graphite is approximately 45 rubles/kg. Finished products are more expensive.
Varieties
Natural graphite is diverse, so a classification has been developed according to several criteria.
By composition and scope of application:
- Colloidal. Technical variety, artificial graphite powder. Used by industry.
- Pyrolytic. Artificial material. Found application as the basis of tools for studying microstructures.
- Siliconized. Silicon enriched graphite. Resistant to corrosion.
Natural graphite according to its structure is divided into fibrous, densely crystalline, scaly, and graphite slate. There are also varieties - graphite and graphite mica.
Recycling
Industrial processing of graphite makes it possible to obtain not only different grades of graphite, but also finished graphite products. Commercial types of this mineral are produced by starting the beneficiation process of graphite-containing ores. Based on the degree of purification, the resulting graphite concentrate will be classified into grades used in industry, as well as by areas of use.
Processing into thermally expanded graphite
First, crystalline graphite undergoes an oxidation process. This process involves the introduction of molecules and ions of nitric or sulfuric acid into the interlayer space of the crystal lattice of the mineral being processed. After oxidation, the graphite undergoes washing and subsequent drying to remove residual water. At the next stage, the mineral undergoes heat treatment at a temperature of 1000 degrees and at a high heating rate. Due to the very rapid heating of the mineral, the process of gas release and decomposition of sulfuric acid molecules embedded in the interlayer space of the crystal lattice of the mineral is triggered.
The release of gaseous substances helps to create an excess disjoining pressure of 400 atmospheres in the intercrystalline space. As a result, thermally expanded graphite is formed, which has a high specific surface area and low bulk density. If sulfuric acid is used to create this artificial mineral, a certain residual amount of sulfur remains in the finished material. At the next stage, the finished thermally expanded graphite is rolled, in some cases additionally reinforced and pressed with the addition of special additives to obtain finished products.
To obtain various grades of artificial graphite
In the process of producing synthetic graphite, petroleum coke is often used, which acts as a structural filler, as well as coal tar pitch, which is used as a binder. In structural types of artificial minerals, soot and graphite of natural origin are used as a special additive. Also, various artificial resins (furan, phenolic, and so on) can be used as a binding component instead of pitch.
The synthetic graphite production process consists of the following technological industrial stages:
- coke is prepared for the production process, it undergoes preliminary crushing by crushing, pierced, and also dispersed into separate fractions;
- the binder is prepared;
- a carbonaceous mass is prepared;
- green unfired blanks are formed into a solid matrix;
- the blanks are fired;
- the process of graphitization of previously fired workpieces is carried out;
- At the final stage, the blanks undergo mechanical processing to obtain the shape of the finished product.
To obtain composite materials
Today, modern industry produces anti-friction carbon materials of such brands as:
- baked antifriction materials of the AO brand;
- graphite and antifriction materials of the AG brand;
- materials with babbitt, tin and lead impregnation;
- graphite plastic materials of the AFGM, AFG-80VS, 7V-2A, KV, KM, AMS brands.
Such materials are made from unpleasant coke that does not undergo a calcination procedure, as well as coal pitch with the addition of natural graphite. In order to increase the density of the material, the technique of impregnation with metals is used.
Artificial graphite
Graphite is synthesized from coke and pitch. These are products of processing coal, petroleum resins, and coal tar. They are exposed chemically and mechanically at high temperatures. The raw materials are pre-sorted, then calcined and impregnated. The result is material of almost absolute purity.
Artificial graphite is used in everything from harmless plastics to nuclear equipment. The most popular brands:
- battery;
- pencil;
- casting;
- lubricating;
- electric coal;
- elemental;
- nuclear.
For each brand and area of use of graphite, the exact proportion of pitch and coke is selected.
It is not difficult to distinguish man-made samples. For example, by triangular shading on planes. Only minerals of natural origin have it.
STRENGTH
Another characteristic that sets graphite apart from other natural minerals. It changes depending on the temperature. For most brands (including artificial types of material), when heated, the tensile strength in bending, compression and tension increases (up to two times). The maximum is achieved at 2200-2800 degrees. If the temperature rises above 3000 degrees, the strength characteristics rapidly drop.
Recrystallized material has the highest strength.
Where is it used?
Graphite is almost universal. There is nothing unusual in this: the necessary characteristics are laid down at the stage of its processing. So, some require increased thermal conductivity, others require electrical conductivity. Still others are interested in the strength properties of graphite.
Taking into account the conditions of the finished product, the mineral is in demand for the following purposes:
- Production of refractory containers.
- Lubrication for smelting steel and alloys.
- Nuclear reactor rods at nuclear power plants and other units.
Souvenir graphite block - Additive to the composition of plastic products, refractories (ceramics, bricks).
- Source code for parts of electrical appliances, bearings, car springs.
- Paint used in industry and in everyday life as a protective coating against rust.
- Raw materials for the production of artificial diamonds.
- Ingredient of medicines, food paraffins, essential substances, alcohols, sugar.
The most famous use of graphite is in the core of pencils.
Moscow scientists have created a medicine from graphite to treat skin diseases.
Application in the food industry
The presented substance is also widely used in the food industry. To do this, it undergoes certain processing during production. The densities of iron, ethyl alcohol, graphite and sugar are, for obvious reasons, different. But the presented material may both contain and be part of certain substances. It is found in paraffins, ethers, alcohol and even sugar.
You can verify this by conducting a simple experiment. First you need to take a piece of sugar. It is placed on a hard lid and covered with a cap (you can use a thimble). The metal covering the sugar is then heated to high temperatures. Over time, acrid smoke will begin to emerge from under the thimble. If you put a match near it, the gas will burn.
When the smoke stops coming out, you can remove the thimble. A black mass remains on the lid. This is coal. It represents the carbon from which graphite is made.
Therapeutic effect
Homeopaths were the first to appreciate graphite. They found that the mineral is suitable for the treatment of skin pathologies (eczema, psoriasis, lichen, etc.).
Today the list has been expanded:
- Metabolic disease.
- Malfunction of the thyroid gland.
- Respiratory tract diseases (rhinitis, bronchial asthma).
- Gastrointestinal problems (gastritis, gastric ulcer, duodenal ulcer, colitis).
- Women's ailments (amenorrhea, chronic inflammation of the ovaries, mastopathy).
- Conjunctivitis, cataracts, stye.
The mineral also “supervises” emotional health. It is prescribed for morning headaches, neurasthenia, apathy, and depression.
How to fool a thermometer?
Not every person, even if he is at puberty, will decide to poison his body for the sake of one day of rest from school. After all, there are small tricks that allow you to achieve high values on the thermometer without the danger of acquiring a disability:
- If there is no one in the room, you can simply heat the measuring device itself by quickly rubbing it or applying it to a warm object. The only disadvantage of this idea is the danger of explosion and spillage of very toxic mercury;
- You can move the strip of mercury by tapping from one or the other end of the thermometer;
- If the thermometer is electronic, then simple tricks will not work with it. You will have to get high values on the screen with the help of pets, which are more warm-blooded than humans;
- Does the measurement take place in front of strangers? It doesn’t matter - you can rub your armpits with pepper, mustard or heating medicinal compounds in advance. Of course, it will hurt, but the desired effect will be achieved;
- Secure the heating pad under your clothes so that it is located at some distance from the thermometer (otherwise the device will show astronomical values).