What is
Magnesium is a metal, an element of D. Mendeleev’s periodic table.
International designation (and formula) – Mg (Magnesium).
Under standard conditions, the metal is light, has good malleability, and has a silvery color.
According to the IUPAC (International Union of Pure and Applied Chemistry) classification, magnesium is classified as an alkaline earth metal.
The structure of the atom (the configuration of the electrons of the outer energy level), and similar chemical and physical properties played a role. Although it reacts sluggishly with water at room temperature.
Story
European scientists experimented with ore in the 18th and early 19th centuries. It was possible to obtain only highly contaminated substances.
The real history of the discovery begins in the mid-19th century, when the Frenchman A. Bussy and the Englishman M. Faraday obtained material of a sufficient degree of purity. Both used molten magnesium chloride. The first reduced the substance with metallic potassium, the second - by electrolysis.
The history of the name of the substance dates back to the times of Asia Minor. Magnesia was the name of a city near which deposits of magnesite were discovered.
In Russia, the term " magnesium " has been used since the mid-19th century.
Metal in nature
Magnesium is among the most common components of the earth's crust: a ton contains 19.52 kg of this element (almost 2%).
The main form of occurrence in nature is deposits of dolomite and magnesite. Brucite, kieserite, bischofite and other minerals are saturated with magnesium.
In addition to mineral deposits, natural sources of metal are:
- Sea water.
- Rapa (saturated brine solution of salts). It is obtained from estuaries and artificially created salt water bodies.
Most regions of the planet have deposits of sedimentary origin:
- Magnesite requires hydrothermal vents.
- Dolomite is found in sedimentary carbonate layers. Deposits are also formed by the influence of hot solutions, rivers, and groundwater on limestone. Its reserves are practically inexhaustible.
- Native magnesium fragments are formed by gas flows. Unlike minerals, they are very rare.
It was first discovered at the end of the 20th century in Russia (Eastern Siberia, banks of the Chona River) and Tajikistan (volcanic lava).
Minerals, deposits
Our hero is so chemically active (simply “promiscuous”) that it is almost impossible to find it in nature in its pure form.
Natural sources of magnesium - minerals:
- brucite;
- kieserite;
- dolomite;
- magnesite;
- bischofite;
- epsomite;
- carnallite.
Fire metal can even be extracted from sea water. Self-sedimented lakes (the water in them is called brine) contain a large amount of mineral salts, including magnesium.
Educational: there are many such lakes in the Astrakhan region. These are Belinsky, Zinzilinsky, Mochagovsky self-sedating lakes (the list goes on).
The largest Russian group of deposits - Satkinskoye - (14 explored) is located in the Chelyabinsk region, near the city of Satka. High purity magnesium ores are concentrated here.
We recommend: MELCHIOR - lost and found
Physico-chemical characteristics
The metal has interesting chemical properties:
- It is indifferent to alkalis, but dissolves when interacting with acids. The process is accompanied by a “fountain” of hydrogen.
- Metal heated in air burns, releasing heat and a bright glow.
- Magnesium powder mixed with active oxidizing agents (like potassium permanganate) creates an explosion.
Magnesium reacts violently to moisture when heated. Therefore, burning magnesium is not extinguished with water.
Under standard conditions (in air), the metal oxidizes, causing the surface to become covered with a protective film. Heating in air up to 600°C can destroy it. The "bare" metal turns into a blinding white flash of flame, forming an oxide and a nitride.
| Properties of the atom | |
| Name, symbol, number | Magnesium (Mg), 12 |
| Atomic mass (molar mass) | [24,304; 24.307] a. e.m. (g/mol) |
| Electronic configuration | [Ne] 3s2 |
| Atomic radius | 160 pm |
| Chemical properties | |
| Covalent radius | 136 pm |
| Ion radius | 66 (+2e) pm |
| Electronegativity | 1.31 (Pauling scale) |
| Electrode potential | −2.37 V |
| Oxidation states | 0; +2 |
| Ionization energy (first electron) | 737.3 (7.64) kJ/mol (eV) |
| Thermodynamic properties of a simple substance | |
| Density (at normal conditions) | 1.738 g/cm³ |
| Melting temperature | 650 °C (923 K) |
| Boiling temperature | 1090 °C (1363 K) |
| Ud. heat of fusion | 9.20 kJ/mol |
| Ud. heat of vaporization | 131.8 kJ/mol |
| Molar heat capacity | 24.90 J/(K mol) |
| Molar volume | 14.0 cm³/mol |
| Crystal lattice of a simple substance | |
| Lattice structure | hexagonal |
| Lattice parameters | a=0.32029 nm, c=0.52000 nm |
| c/a ratio | 1,624 |
| Debye temperature | 318K |
| Other characteristics | |
| Thermal conductivity | (300 K) 156 W/(m K) |
| CAS number | 7439-95-4 |
The utilitarian properties of a metal are determined, among other things, by the degree of purity.
Particularly pure samples are ductile, forgeable, and easy to process (pressing, rolling, cutting).
Magnesium. Description and properties of magnesium
Strength, attraction, power - this is how the people of ancient Greece interpreted the word “magnes”. In this country there was a city called Magnesia. Near these settlements, magnetic iron ore was mined, which, as is known, has the power to attract metal objects.
But, the metal magnesium is named not after the iron-containing rock, but after the powder “white magnesia”. The Greeks obtained it from a mineral that was also available near the ancient settlement. After calcination, the stone turned into white powder - magnesium oxide . The Greeks did not know that the substance was metal, but they noticed the healing properties of the composition. It helped with liver and kidney diseases, and played the role of a laxative.
The drug did not go out of use for centuries and, in 1808, Geoffrey Davy isolated an unknown metal from it during experiments. Without thinking for long, a scientist from England named the discovered element magnesium. This is what it is still called in Europe. Russians call the metal magnesium thanks to the textbook by Hermann Hess. Despite his German roots, the chemist is Russian. In 1831 he translated a Western textbook. The scientist transformed the word “magnesium” into “magnesium”. This is how the element received a special name in Russian science.
In the periodic table of chemical elements, Magnesium occupies the 12th position. It is located in the main subgroup of the group at number two. The element is white with silver reflections. This color is characteristic of all alkali earth metals, which, along with strontium, radium and barium, also includes magnesium. It is “fluff” among metals. For example, iron and copper are almost 5 times heavier. Even lightweight aluminum will outweigh element No. 12 on the scales.
The lightness of magnesium is beneficial to aircraft designers and manufacturers. They don't have to be heavy to have good flying properties. However, pure metal No. 12 cannot be used for the same aircraft. He is too soft and pliable.
It is necessary to make alloys with manganese , aluminum or zinc. They give manganese strength without adding much weight. The mixtures are used mainly for the production of cladding for “iron birds”. The first aircraft based on magnesium alloys, by the way, was the work of domestic aircraft technicians. The ship was created back in 1934 and named “Sergo Ordzhonekidze”.
The element magnesium is very difficult to melt down. Only 650 degrees Celsius is required. However, already at 550 the metal flares up and dissolves in the atmosphere. The flame produced is very impressive, so the metal has found application in the pyrotechnic industry.
Not a single firework or sparkler is complete without it. If magnesium is stored at home, it is better not to spill bleach near it. In the presence of chlorine, the 12th element lights up even at a temperature of 25 degrees.
The combustion products of magnesium are ultraviolet rays and heat. Even a few grams of metal is enough to boil 200 milliliters of water. This is quite enough to drink tea. Scientists from Warsaw decided to “force” the element to heat food. magnesium tape into canning jars . When the container is opened, the insert ignites, heating the contents of the jar. Here's a ready-made lunch.
Self-heating cans have been in production for thousands of years. Deposits of magnesium in the depths compete with reserves of only 7 elements. There is only more silicon, oxygen, iron, aluminum and calcium. Metal number 12 is part of two hundred minerals. The element is extracted on an industrial scale from dolomite and carnallite.
Magnesium is also a major component of magma, the hot layer between the planet's core and its surface. In sea water, element No. 12 contains 4 kilograms per cubic meter.
If ocean water is mixed with shells crushed into powder, magnesium chloride . Pure metal can be isolated from it by electrolysis. But this method was used only during the Second World War. We extracted about 100 thousand tons of element No. 12 and calmed down, because processing the resources of the seas in huge tanks is a troublesome business.
For metallurgy, one of the main consumers of magnesium, there are enough reserves in the earth’s crust. The metal is necessary in the production of almost all alloys. Element No. 12 reduces the oxygen content in them, which sharply worsens the quality of the product. Getting magnesium to become part of any alloy is not easy. Due to its lightness, it does not sink in other metals. Due to the “explosive reaction” to air, it flares up on the surface of the mixtures.
Metallurgists have to press capricious metal into briquettes, place weights inside them and, only then, lower them into the composition for remelting.
The lightness of magnesium also attracted jewelers. They add the element to precious alloys to make the items lighter. This is very useful if the decoration is voluminous and of impressive dimensions. Not everyone wants to carry an incredible weight for a piece of jewelry. Magnesium comes to the rescue.
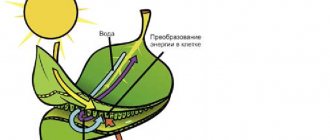
But, if jewelry making without magnesium is possible, then life is not. The metal magnesium is part of chlorophyll. It is part of vegetation, a substance responsible for photosynthesis. That is, without element No. 12, the process of converting carbon dioxide into oxygen would be impossible. The atmosphere of the planet would be different, so humanity would hardly have appeared on Earth if there were no magnesium on it.
This metal also helps the human heart beat, not only by supplying it with oxygen. Magnesium is necessary for stable functioning of the heart muscle. According to statistics, heart attacks occur mainly in people whose bodies do not have enough element No. 12. Therefore, it won’t hurt to eat pumpkin seeds, bran, drink cocoa and tea. These foods contain the most magnesium.
Metal production technology
In the third millennium, metal is obtained in the same way as two hundred years ago.
Electrolysis
The main method of obtaining the substance remains the electrolysis of a molten conglomerate of magnesium, potassium, and sodium chlorides:
- The object of electrochemical reduction is a magnesium compound.
- The process takes place in the bath. Portions of released metallic magnesium are periodically scooped out from it.
The purity of the product at the exit is about 99.9%.
- It is considered “dirty”, so it is again sent to electrolysis for purification (refining).
- Next comes vacuum remelting, during which special additives are added to the material. Their mission is to draw impurities towards themselves.
- As an option, vacuum distillation is used.
The proportion of impurities decreases by two orders of magnitude. That is, the purity of magnesium metal is 99.999+%.
Heat treatment
Magnesium oxide is reduced at high temperatures using coke or silicon.
Silicon is able to “pull” metal out of complex types of raw materials. For example, dolomite - a conglomerate of calcium and magnesium carbonates.
First, dolomite is fired, then the resulting products are heated with silicon.
In this way, it is possible to extract high-purity metal even from sea water or brines.
Electrical conductivity - aluminum - Great Encyclopedia of Oil and Gas, article, page 4
Conductivity - Aluminum
Page 4
A more important property of coatings when used as electrical contacts is their high hardness and the ability to remain free of oxide films and dull deposits. Thus, although aluminum has four times the electrical conductivity of tin, it is often coated with a layer of tin to improve electrical contact properties. Other metal platings commonly used for low voltage equipment are gold, tin-lead, silver, palladium, copper, rhodium and nickel. [46]
Copper conductors are made from annealed wire to increase electrical conductivity. Aluminum conductors do not require annealing, since the electrical conductivity of aluminum does not change depending on the degree of annealing. [47]
The conductive cores of power cables are made of copper or aluminum wire. Since the electrical conductivity of copper is approximately 1.65 times higher than the electrical conductivity of aluminum, for the same load the cross-section of aluminum conductors should be correspondingly larger than the cross-section of copper conductors. Copper conductors have higher mechanical strength than aluminum ones. The advantages of aluminum as a material for conductive conductors is that the specific gravity of aluminum is 3 3 less than the specific gravity of copper, and therefore, with the same electrical conductivity, the weight of the conductors is approximately 2 times less than the weight of copper conductors. In addition, the advantage of aluminum cores is the low cost and lower scarcity (compared to copper) of aluminum. [48]
Lithium in alloys contributes to an increase in their elastic modulus. At the same time, magnesium and manganese reduce the heat and electrical conductivity of aluminum, and iron reduces its corrosion resistance. [49]
Magnesium is of medium hardness and is quite malleable, so that when rolled it can be used to produce thin sheets and wire; however, these properties change significantly under the influence of very small impurities. The electrical conductivity of magnesium is 9/25 of the electrical conductivity of copper and 12/19 of the electrical conductivity of aluminum, and the thermal conductivity is 5/13 of the thermal conductivity of copper and 7/d of the corresponding value for aluminum. Cold water acts on magnesium extremely slowly; Nevertheless, when water acts on defatted magnesium shavings (washed in ether), the rising gas bubbles can be used to judge the evolution of hydrogen. With boiling water this reaction proceeds much more vigorously. [50]
Pages: 1 2 3 4
www.ngpedia.ru
Where is it used?
The metal's most famous use was once in the magnesium flash camera. Today, the properties and characteristics of the substance have made it indispensable or important for many industries. Magnesium metal is used in compounds and alloys.
Magnesium alloys
Industrial complex
Malleable, ductile, lightweight magnesium is one of the metals that is indispensable in metallurgy and mechanical engineering:
- Refractory materials are the source for the production of components of metallurgical furnaces (magnesium oxide).
- Raw materials for producing reliable light and ultra-light alloys. They are taken by the rocket, aircraft, and automobile industries.
- An innovative material created at the end of the 20th century is ferrosilicon magnesium (magnesium + silicon + iron). Cast iron is smelted from this alloy: high-strength and with a “worm” graphite configuration. This is a kind of hybrid of cast iron and steel, a new generation material for the needs of mechanical engineering.
In warfare, the property of metal to burn with a blinding flame was useful.
Flash-noise ammunition (incendiary bombs, signal flares, tracer bullets) is purchased.
Other industries
Magnesium is used for the needs of science and other industries:
- Energy-intensive backup electric batteries and dry elements based on metal and its compounds (bromide, perchlorate) have become a common attribute.
- Grown magnesium fluoride crystals are a component in the manufacture of lenses and other optics.
- Laboratory assistants dry gases with metal perchlorate.
- Metal bromide serves as an electrolyte for chemical current sources.
Without magnesium components it is impossible to manufacture pyrotechnics.
Compounds of the substance are in demand in medicine. Cardiologists, neurologists, and gastroenterologists prescribe magnesium sulfate/citrate, asparkam, and other drugs to their patients.
Prospects
Scientists are working on creating magnesium-sulfur batteries. According to calculations, they are more efficient than lithium-ion batteries in terms of capacity.
Application
In recent years, the demand for non-ferrous metals has increased sharply. They influence the development of many industries and are widely used in aircraft and mechanical engineering, radio electronics, rocket and nuclear technology, high technology, as well as in everyday life.
Non-ferrous metals are an irreplaceable raw material in the production of rolled metal, large structures and small products.
You can order non-ferrous metals and alloys on our website. The catalog page presents a wide range of products with detailed descriptions and prices. The cost per 1 kg depends on the type of material and varies from 135 to 2200 rubles. We accept funds to the bank account. Read more about the conditions for purchasing non-ferrous metals in Moscow and Russian regions here.
World production, prices
In the 20th century, half of the pure magnesium was smelted in the USA. The third millennium determined a new leader - China (70% of world production).
Magnesium metal
Next come Russia and Türkiye (both receive an order of magnitude less product).
The price of metal on the world market is determined by the state of the Chinese economy.
By the beginning of 2021, $2,400-2,600 were paid per ton of metal.
Groups of non-ferrous metals
Non-ferrous metals are divided into the following groups: heavy metals, light metals, noble metals, minor metals, refractory metals, trace metals, radioactive metals.
Groups of non-ferrous metals
Non-ferrous metals are divided into the following groups:
- heavy metals - copper, nickel, zinc, lead, tin
- light metals - aluminum, magnesium, titanium, beryllium, calcium, strontium, barium, lithium, sodium, potassium, rubidium, cesium
- noble metals - gold, silver, platinum, osmium, ruthenium, rhodium, palladium
- minor metals - cobalt, cadmium, antimony, bismuth, mercury, arsenic
- refractory metals - tungsten, molybdenum, vanadium, tantalum, niobium, chromium, manganese, zirconium
- rare earth metals - lanthanum, cerium, praseodymium, neodymium, samarium, europium, gadolinium, terbium, ytterbium, dysprosium, holmium, erbium, thulium, lutetium, promethium, scandium, yttrium
- trace metals - indium, germanium, thallium, thallium, rhenium, hafnium, selenium, tellurium
- radioactive metals - uranium, thorium, protactinium, radium, actinium, neptunium, plutonium, americium, californium, einsteinium, fermium, mendelevium, nobelium, lawrencium
Non-ferrous metals and their alloys are widely used in technology. The most important non-ferrous metals include aluminum and aluminum alloys (duralumin), copper and its alloys - bronze and brass, magnesium, nickel, titanium and soft metals - tin, lead and zinc. Metals often used in alloys include antimony, bismuth, cadmium, mercury, cobalt, chromium, molybdenum, tungsten and vanadium. The last four metals are conventionally classified as ferroalloys, although they may contain iron only as an impurity.
Meaning for humans
Magnesium is initially present in biological organisms. However, in everyday products (bread, milk, meat) there is a minimum of it.
Life processes
Without metal, the normal course of important life processes is impossible:
- Protein synthesis.
- The work of the nervous system and heart muscle.
- Vasodilation.
- Bile secretion.
- Work of the gastrointestinal tract.
- Removing cholesterol from the body.
- Muscle contraction.
The daily intake of the substance for women and men is 300 and 400 mg.
The need is increased by mental and physical overload, stress, alcohol abuse, and sweating.
It must be taken into account that the body absorbs only a third of the total amount of the substance ingested.
Phytin, fatty or calcium-rich diets interfere with the absorption of microelements more fully.
Nutrition
Cocoa powder, bran, nuts, and pumpkin seeds are saturated with magnesium. However, the abundance of phytin in them interferes with the absorption of the element.
Nutritionists consider green vegetables to be a storehouse of magnesium. These are cabbage, cucumbers, peas, asparagus, celery, onions, spinach, parsley.
Consequences of deficiency or excess of a substance
Symptoms of magnesium deficiency:
- Bone problems (arthritis, osteoporosis).
- Cramps, muscle spasms.
- Headache.
- Malfunctions of the gastrointestinal tract (constipation), heart (arrhythmia).
On the emotional level, a lack of substance leads to insomnia, permanent fatigue, irritability (especially PMS).
Excessive interest in microelements is also dangerous.
An overdose of metal is signaled by:
- Reduced blood pressure.
- Nausea, vomiting.
- Depression of the central nervous system, reflex function, breathing.
The case may end in coma, paralysis of the respiratory tract, and heart muscle.
Warning
The toxicity of metal compounds is insignificant. The only dangers are hydrocyanic, hydrofluoric, chromic, and hydronitric acids.
Dangerous characteristics of magnesium are also associated with combustion:
- Contemplation of a burning substance leads to retinal burns and temporary blindness. Insurance - look through dark glasses or glass.
- There is no insurance against tactile contact with metal. The rate of ignition of the substance is such that a person is guaranteed not to have time to remove his hand and gets burned.
Caution when taking magnesium supplements is required for people suffering from kidney failure.
Interaction with various acids
For brevity, it's easier to look at a few experiments. The following types of acids are used for them:
- Solyanaya.
- Nitrogen.
- Sulfuric (diluted and not).
In the first case, almost instantaneous dissolution is observed, accompanied by bubbles of white gases and a pungent odor of chlorine. The container in which the reaction took place heats up.
A piece of magnesium does not sink in nitric acid. Brown gas accumulates above the surface of the liquid and heat is released. The acid is sometimes said to be "boiling" around the magnesium pieces.
The third case must be considered as two particular ones. In undiluted sulfuric acid the reaction proceeds slowly. If you use a solution with a small amount of water, magnesium, just like with nitric acid, floats on the surface. In this case, a barely noticeable reaction occurs with the release of white gas bubbles.