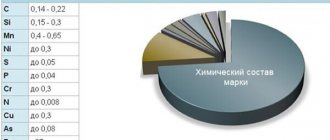
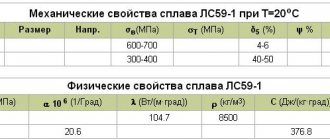
Density of structural alloy steel
Structural alloy alloys are used in the production of highly loaded critical structures, including those operating in aggressive environments. The density of grade 30KhGSA is close to the standard value of 7.85 t/m3, the density of low-alloy structural steel for welded structures
Low-alloy alloys have excellent weldability and high corrosion resistance, so they are widely used for critical structures in construction and shipbuilding. The HC of steel in this group ranges from 7.85-7.87 t/m3 and is given in the table:
| Group | Brand | Density |
| low alloy structural | 09G2S | 7,85 |
| high carbon | 70 (VS and OVS) | 7,85 |
| medium carbon | 45 | 7,85 |
| low carbon | 10, 10A, 20, 20A | 7,85 |
| carbon structural | St3sp, St3ps | 7,87 |
W=(mvl- mc)/mc*100
where, mvl, mc is the mass of wet and dry material.
Water permeability is the ability of a material to pass water under pressure. The water permeability of a material depends on its porosity and the nature of the pores. Water permeability is encountered during the construction of hydraulic structures and water tanks.
The inverse characteristic of water permeability is water resistance - the ability of a material not to allow water to pass under pressure. Very dense materials (steel, bitumen, glass) are waterproof.
Frost resistance is the ability of a material in a water-saturated state to withstand repeated alternating freezing and thawing without signs of destruction and without a significant decrease in strength.
Destruction occurs due to the fact that the volume of water when turning into ice increases by 9%. The pressure of ice on the pore walls causes tensile forces in the material.
The frost resistance of materials depends on their density and the degree of filling with water.
Samples of the tested material, depending on their purpose, must withstand from 15 to 50 or more freezing and thawing cycles. In this case, the test is considered passed if there are no visible damages on the samples, the loss in weight does not exceed 5%, and the decrease in strength does not exceed 25%.
Frost resistance is of great importance for wall materials that are alternately exposed to positive and negative temperatures, and is measured in freeze-thaw cycles.
Thermal conductivity is the ability of a material to conduct heat. Heat transfer occurs as a result of temperature differences between the surfaces bounding the material.
The greater the porosity and the lower the average density, the lower the thermal conductivity coefficient. This material has greater thermal resistance, which is very important for external enclosing structures (walls and coverings). Materials with a low thermal conductivity coefficient are called thermal insulation materials (mineral wool, polystyrene, foam concrete, polystyrene concrete, etc.) They are used to insulate walls and coatings. The most thermally conductive materials are metals.
The thermal conductivity of materials increases significantly with moisture. This is explained by the fact that the thermal conductivity coefficient of water is 0.58 W/(m*oC), and that of air is 0.023 W/(m*oC), i.e. exceeds it by 25 times. Thermal conductivity coefficients of individual materials are given in Table 1.
Fire resistance is the ability of materials to maintain their strength under high temperatures. Ignition resistance is determined by the degree of flammability. Based on the degree of flammability, building materials are divided into non-combustible, non-combustible and combustible.
Polystyrene concrete is a low-flammable material and has a flammability group of G1. Cellular concrete is not a combustible material.
Fireproof materials do not ignite, smolder or char. These include stone materials (concrete, brick, granite) and metals.
Refractory materials ignite with great difficulty, smolder or char only in the presence of a fire source, for example, fiberboard boards, gypsum products with organic filling in the form of reeds or sawdust, felt soaked in a clay solution, etc. When the fire source is removed, these processes stop.
Combustible materials are capable of igniting and burning or smoldering after the fire is removed. All unprotected organic materials (timber, reeds, bitumen materials, felt and others) have such properties.
Fire resistance is the ability of a material to withstand prolonged exposure to high temperatures without melting or softening. According to the degree of fire resistance, materials are divided into the following groups: fire-resistant, refractory and low-melting. Fireproof ones can withstand temperatures of 1580°C and above, refractory ones - 1350 - 1580°C, low-melting ones - less than 1350°C. Refractory materials are used in the construction of industrial furnaces, for lining boilers and thermal pipelines (refractory bricks, heat-resistant concrete, etc.).
Density of stainless steel
The density of a substance is calculated by dividing the mass of an object by its volume. Such calculations have already been made for all substances known to man, and metrological services periodically repeat and refine these measurements. In practice, people face another practical task: knowing the material from which the product is made, determine its mass.
The density of a substance is also called specific gravity (or, in everyday life, specific gravity) - that is, the mass of a solid physical body made of a given substance and having a unit volume.
Stainless steel
It should be noted that when using the term “mass”, in 99% of cases people are dealing with weight - the force of attraction of the physical body to the Earth. The fact is that to determine body weight in a strict physical sense, sophisticated equipment is required, available only in the largest scientific centers. For practical use, in most cases, conventional, more or less accurate scales using the Earth's gravity and springs, or levers and standard weights, or piezoelements are sufficient.
In practice, to calculate the weight of a linear or square meter of rolled metal, the specific gravity, or density of the material from which it is made, is used. In reference books on the assortment of rolled metal, among the main characteristics of each grade, the mass of a linear or square meter and the density value used in the calculations must be indicated.
In most cases, calculations based on the mass of a linear or square meter are sufficient for practical applications. Raw materials and components are purchased with a certain standardized stock, and before shipment to the consumer, the product is weighed on scales for accurate settlements between contractors.
However, you need to understand that the data in the directory is calculated based on the standard density of steel, most often it is 7.85 t/m3. At the same time, the actual density of a particular steel grade depends on the composition and specific amount of additives and can range from 7.6 to 8.8 t/m3.
This can give an error of up to 10% up or down for a product made from a very light or, conversely, very heavy alloy. For a small amount of metal the difference will be small and can be neglected. However, for complex products that use large volumes of metal, more accurate calculations will be required.
The mass will be needed when creating an application for the purchase of metal. Based on the density of a given alloy, an adjustment is made to the reference values of the mass of one linear or square meter, and then the already specified value is used in the calculations.
How to calculate P or perform 1 meter mass adjustment?
The practical method for determining density is quite simple and is known to us from a school physics course. A sample of material is lowered into a measuring container filled with water to a certain level. The water level rises to a certain height. The volume of displaced water is equal to the volume of the sample. The mass of the sample is determined by weighing on an accurate balance. Density will be equal to the ratio of mass and volume.
To adjust the mass of a linear or square meter, you need to divide the value from the reference book by the density from the reference book and multiply the result by the measured density of the sample material. The corrected value will be obtained.
If similar calculations are expected to be repeated, then it will be more convenient to calculate a correction factor equal to the ratio of the standard density and the density of the sample, and then apply it in the calculations.
Density of 12Х18Н10Т and some stainless steels
Grade 12×18N10T is one of the most widely used stainless steels. The density for it and several popular brands in production is given in the table, the brands are arranged in order of increasing density. The third column shows the density adjustment factor relative to the standard value of 7.85:
| steel grade | Density t/m3 | Correction factor |
| 08Х22Н6Т 15Х28 | 7,60 | 0,97 |
| 08Х13 12Х17 | 7,70 | 0,98 |
| 04Х18Н10 08Х18Н12Б 12Х18Н10Т 17Х18Н9 | 7,90 | 1,01 |
| 08Х18Н12Т 10Х23Н18 | 7,95 | 1,01 |
| 06ХН28МДТ 08ХН28МДТ | 7,96 | 1,01 |
| 10Х17Н13М2Т | 8,00 | 1,02 |
| 08Х17Н15М3Т | 8,10 | 1,03 |
Determination of the mass of 1 pm of rolled metal
| Determination of the theoretical mass of 1 linear meter of pipe |
For stainless steel pipe: m = ?*(d – s)*s*?/1000
For the “black” pipe: m = (d – s)*s/40.55
where: m – theoretical. weight of one linear meter of pipe in kg, ? = 3.14 (constant value), d – outer diameter in mm, s – wall thickness in mm, ? – density in g/cub. cm.
| Determination of the theoretical mass of 1 linear meter of a circle |
m = ?*d2*?/4000
where: m – theoretical. mass of 1 p/m of circle in kg, ? = 3.14 (constant value), d – outer diameter in mm, ? – steel density in g/cubic meter. cm.
| Determination of the theoretical mass of one sheet |
m = V* ?/ 1E 6
where: m – theoretical. weight of 1 p/m sheet in kg, V – sheet volume = Thickness x Width x Length, mm, ? – steel density in g/cubic meter. cm, 1E6 – the number 10 to the 6th power.
| Determining the approximate number of sheets in one ton |
n = 1E 9 / V*?
Where: ? – steel density in g/cubic meter. cm V – sheet volume = Thickness x Width x Length, mm,
Density of different steel grades (according to GOST 9941-81)
| steel grade | Density, ?, g/cm3 | steel grade | Density, ?, g/cm3 |
| 04Х18Н10 | 7,90 | 08Х18Н10 | 7,90 |
| 08Х20Н14С2 | 7,70 | 08Х17Т | 7,70 |
| 10Х17Н13М2Т | 8,00 | 08Х13 | 7,70 |
| 08Х18Н12Б | 7,90 | 12Х13 | 7,70 |
| 10Х23Н18 | 7,95 | 12Х17 | 7,70 |
| 08Х18Н10Т | 7,90 | 15Х25Т | 7,60 |
| 08Х18Н12Т | 7,95 | 12Х18Н9 | 7,90 |
Density of other steels and alloys
The specific gravity of steel of other groups is given in the table:
| Steel type | Brand | Density |
| cryogenic stainless structural | 12Х18Н10Т | 7,9 |
| heat-resistant stainless steel corrosion-resistant | 08Х18Н10Т | 7,9 |
| stamped instrumental | X12MF | 7,7 |
| stamped instrumental | 5ХНМ | 7,8 |
| low-carbon electrical (Armco) | A and E; EA; EAA | 7,8 |
| chromium | 15ХА | 7,74 |
| chrome-aluminium-molybdenum nitrided | 38ХМУА | 7,71 |
| chromium-manganese-silicon | 25ХГСА | 7,85 |
| chrome vanadium | 30ХГСА; 20ХН3А | 7,85 |
Density of pure metals
| Name of material, brand | Density ρ, kg/m3 |
| Aluminum | 2700 |
| Beryllium | 1840 |
| Vanadium | 6500-7100 |
| Bismuth | 9800 |
| Tungsten | 19300 |
| Gallium | 5910 |
| Hafnium | 13090 |
| Germanium | 5330 |
| Gold | 19320 |
| Indium | 7360 |
| Iridium | 22400 |
| Cadmium | 8640 |
| Cobalt | 8900 |
| Silicon | 2550 |
| Lithium | 530 |
| Magnesium | 1740 |
| Copper | 8940 |
| Molybdenum | 10300 |
| Manganese | 7200-7400 |
| Sodium | 970 |
| Nickel | 8900 |
| Tin | 7300 |
| Palladium | 12000 |
| Platinum | 21200-21500 |
| Rhenium | 21000 |
| Rhodium | 12480 |
| Mercury | 13600 |
| Rubidium | 1520 |
| Ruthenium | 12450 |
| Lead | 11370 |
| Silver | 10500 |
| Waist | 11850 |
| Tantalum | 16600 |
| Tellurium | 6250 |
| Titanium | 4500 |
| Chromium | 7140 |
| Zinc | 7130 |
| Zirconium | 6530 |
Steel - concept and its characteristics
Steel is the most common material for the manufacture of structures, parts, mechanisms and tools. Steels include all alloys of iron and carbon, and the share of iron must be at least 45%, and the share of carbon - less than 2.14 percent. Carbon, lining up in the molecular structures of iron, increases strength and hardness, but makes the alloy less ductile and malleable. In addition to carbon, the alloy contains metals and non-metals. The most important characteristics of the alloy include:
- shear modulus;
- elastic modulus;
- density;
- linear expansion coefficient.
Different areas of application of materials require them to have different physical and chemical properties. For example, steel alloys with a high modulus of elasticity are used for the production of springs and spring-type shock absorbers. These properties are purposefully changed as a result of the addition of various additives.
Melting steel
The density of steel, or HC steel, is one of the most important characteristics of the alloy. Based on it, the designer calculates the weight of the part and the total weight of the product, logistics organizes the purchase and delivery of raw materials, blanks and finished products, economists determine the cost. The weight of steel is determined as the product of density and volume.
Steel classification
Depending on the proportion of non-metallic impurities determined by the method of smelting a given grade, steel alloys are divided into:
- especially high quality;
- high quality;
- ordinary quality.
Based on their chemical composition, alloys are also divided into alloyed and carbon.
Carbon steels
Used primarily for the production of welded structures and contains from 0.25 to 2.14 percent carbon. Within the group, they are further divided into subgroups, and also according to the percentage of carbon:
- high carbon (0.6-2.14);
- medium carbon (0.3-0.55);
- low carbon (below 0.25).
They also contain silicon and manganese as additives. In addition to useful, purposefully introduced additives, the alloy may also contain harmful impurities that negatively affect its physicochemical properties:
- phosphorus reduces ductility when heated and increases brittleness when cooled;
- sulfur leads to the formation of microcracks.
Low carbon steel
Other impurities may also be present in the alloy.
Mechanical and technological characteristics of steel
It is very difficult to determine the specific physical and mechanical properties of steel, since the number of its types is varied due to different compositions and heat treatments, which allow the creation of materials with a wide variety of chemical and mechanical characteristics. This diversity has led to the fact that the production of these materials and their processing began to be separated into a separate branch of metallurgy - ferrous metallurgy, which differs from non-ferrous metallurgy. However, general properties for steel can be given; they are presented in the list below.
- The volumetric weight of steel, that is, the mass of 1 m³, is 7850 kg. The density of steel g cm3 is therefore 7.85.
- Depending on the temperature, the material can be bent, stretched and melted.
- The melting point depends on the type of alloy and the percentage of additives. Thus, pure iron melts at a temperature of 1510 °C, in turn, steel has a melting point equal to 1375 °C, which increases as the percentage of carbon and other elements in it increases (the exception is eutectics, which melt at lower temperatures). High-speed steel melts at a temperature of 1650 °C.
- The material boils at a temperature of 3000 °C.
- It is a deformation-resistant material whose hardness increases with the addition of other elements.
- It has relative malleability (it can be used to produce thin threads by drawing - wire), as well as ductility (you can produce flat metal sheets 0.12-0.50 mm thick - tin, which is usually coated with tin to prevent oxidation).
- Before using thermal treatment, the alloy undergoes mechanical processing.
- Some composites have shape memory and deform by an amount exceeding the yield strength.
- The hardness of steel varies between the hardness of iron and the hardness of structures that are obtained through thermal and chemical processes. Among them, the best known is hardening, which is applied to materials with a high carbon content. The high surface hardness of steel allows it to be used as a cutting tool. To obtain this characteristic, which is maintained at high temperatures, chromium, tungsten, molybdenum and vanadium are added to the steel. The hardness of metal is measured using Brinell, Vickers and Rockwell.
- Has good casting properties.
- The ability to corrode is one of the main disadvantages of steel, since oxidized iron expands in volume and leads to cracks on the surface, which in turn further accelerates the process of destruction. Traditionally, metal was protected from corrosion using various surface treatments. In addition, some steel compounds are resistant to oxidation, such as stainless steel materials.
- It has high electrical conductivity, which does not vary much depending on the composition of the alloy. In overhead power lines, aluminum conductors are most often used, which are covered with a steel jacket. The latter provides the necessary mechanical strength to the wires, and also contributes to their cheaper production.
- Used to produce artificial permanent magnets because magnetized steel does not lose its magnetic ability up to a certain temperature. In this case, the ferrite structure of steel has magnetic properties, while the austenite structure is not magnetic. To stabilize the ferrite structure, steel-based magnets usually contain about 10% nickel and chromium.
- With increasing temperature, a product made of this material increases its length. Therefore, if there are degrees of freedom in a particular structure, then thermal expansion is not a problem, but if such degrees of freedom do not exist, then the expansion of the steel will lead to additional stresses that must be taken into account. The coefficient of thermal expansion of steel is close to that of concrete. This fact makes it possible to use them together in structures of various types; this material is called reinforced concrete.
- It is a non-flammable material, but its fundamental mechanical properties quickly deteriorate when exposed to open flame.