In order to increase the strength and hardness of materials, they are subjected to heat treatment: they are heated and kept in a thermal oven and cooled. But this method is not always suitable. In particular, it is not used for metals such as copper and aluminum.
Then cold hardening is used - a technological treatment that involves changing the shape of the product through cold plastic deformation. At the same time, the hardness and strength of the material increases, but its ductility - the ability to deform without destruction - decreases.
Cold hardening and cold hardening
For some alloys, cold hardening is the only possible way to increase strength. Such alloys, for example, include corrosion-resistant alloys of chromium and nickel.
The study of a process such as cold hardening (hardening of metal) is one of the important and interesting problems of materials science. For example, as a result of cold hardening, the hardness of the surface layers of steel increases several times.
The terms cold hardening and cold hardening are often considered almost synonymous, meaning:
- the process of changing the structure of the material;
- increasing its hardness and strength as a result of these changes.
But in some literary sources these terms are distinguished: cold hardening is a process that can be either spontaneous or purposeful, while cold hardening is a deliberate process, the purpose of which is to strengthen the metal.
From this point of view, cold hardening can be a process both useful and harmful, and cold hardening is a process that can only be useful.
As the temperature increases, the cold hardening ability decreases noticeably. For example, cold hardening of aluminum is impossible at temperatures above 200 °C. This temperature (recrystallization temperature) will be different for different substances. For low-melting metals (including zinc, lead, tin), the recrystallization temperature can be negative.
Process description
Let us consider the essence of the phenomenon of cold hardening. As is known, almost all metals and their alloys (for example, aluminum or copper and their alloys) have an ordered crystalline structure. But it's not that simple. They consist of grains, within which the arrangement of atoms is ordered. But the grains themselves are located chaotically in relation to each other, that is, disordered.
Under mechanical load, dislocations (microscopic defects) appear in the structure of the substance. As the load increases, dislocations move and interact with each other.
Another structure is formed. It resists the deformation that remains after the load is removed (plastic deformation). The ability of the metal to resist deformation increases.
But it should be borne in mind that with cold hardening, the plastic properties of the material become worse. For example, the ductility of low-carbon steel decreases by 5-6 times. The resistance to plastic deformation also decreases when its sign changes (the so-called Bauschinger effect).
After hardening, the state of the substance is thermodynamically unstable. If ductility needs to be increased, hardening is removed by recrystallization annealing, heating the material above the recrystallization temperature. In this case, the material goes into a more stable state. The need to remove hardening arises, for example, in metallurgy during the production of wire or tape.
The dislocation density increases during work hardening, which leads to a decrease in bulk density. In this case, the metal grains are stretched in the direction of the forces that act on them. This grain orientation is called deformation texture. Due to texture, anisotropy of the mechanical properties of metals and alloys occurs.
The following conclusions can be drawn:
- after cold hardening or hardening, the hardness and strength of the material increases;
- The fragility of the material also increases.
In particular, cold hardening of steel is relevant for products in which it is necessary to prevent surface cracking and such phenomena as metal fatigue, which leads to the accumulation of internal stresses, the occurrence of cracks, and ultimately to the destruction of the material.
Types of hardening
Basically, there are two types of cold hardening:
- phase, when changes in the crystal lattice are caused by phase changes;
- deformation, when lattice changes are caused by external forces.
The formation of deformation hardening occurs when the surface being treated is exposed to balls or a stream of pellets.
Hardening equipment
Equipment for the process of cold hardening of aluminum and other metals and alloys is quite diverse. In industry, cold hardening is a fully automated process that is performed on electronically controlled devices.
In particular, when deformation hardening is formed, the number and feed rate of pellets is automatically adjusted.
Application
In industry, cold hardening is used to impart strength to products made of stainless steel, copper, aluminum and its alloys. This is very important for mechanical engineering, since various components and mechanisms often operate in unfavorable conditions and wear out over time.
Metal hardening methods
One of the technological processes of hardening treatment is thermomechanical treatment
(TMO)
.
Thermo-mechanical treatment
refers to combined methods of changing the structure and properties of materials. Thermo-mechanical processing combines plastic deformation and heat treatment (hardening of pre-deformed steel in the austenitic state).
The advantage of thermomechanical processing is that with a significant increase in strength, the ductility characteristics decrease slightly, and the impact strength is 1.5 - 2 times higher compared to the impact strength for the same steel after quenching with low tempering.
Depending on the temperature at which deformation is carried out, a distinction is made between high-temperature thermomechanical treatment (HTMT) and low-temperature thermomechanical treatment (LTMT).
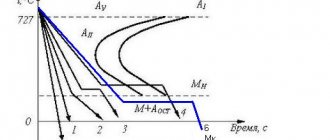
The essence of high-temperature thermomechanical treatment is to heat the steel to the temperature of the austenitic state (above A3). At this temperature, the steel is deformed, which leads to hardening of austenite. Steel with this state of austenite is subjected to hardening (Fig. 5.16, a
).
a b
Rice. 5.16. Scheme of thermomechanical processing modes of steel:
A
– high-temperature thermomechanical treatment (HTMT);
b
– low temperature thermomechanical treatment (LTMT)
High-temperature thermomechanical processing virtually eliminates the development of temper brittleness in the dangerous temperature range, weakens irreversible temper brittleness and dramatically increases impact strength at room temperature. The temperature threshold for cold brittleness decreases. High-temperature thermomechanical treatment increases resistance to brittle fracture and reduces sensitivity to cracking during heat treatment. High-temperature thermomechanical processing can be effectively used for carbon, alloy, structural, spring and tool steels. Subsequent tempering at a temperature of 100–200 °C is carried out to maintain high strength values.
Low-temperature thermomechanical processing (ausforming).
The steel is heated to an austenitic state. Then it is kept at a high temperature, cooled to a temperature above the temperature of the onset of martensitic transformation (400 - 600 oC), but below the recrystallization temperature, and at this temperature pressure treatment and quenching are carried out (Fig. 5.16, b
).
Low-temperature thermomechanical treatment, although it gives higher strengthening, does not reduce the tendency of steel to temper brittleness. In addition, it requires high degrees of deformation (75 – 95%), so powerful equipment is required.
Low-temperature thermomechanical processing is applied to martensite-hardened medium-carbon alloy steels that have the secondary stability of austenite.
The increase in strength during thermomechanical treatment is explained by the fact that as a result of deformation of austenite, its grains (blocks) are crushed. The dimensions of the blocks are reduced by 2–4 times compared to conventional hardening. The dislocation density also increases. With subsequent quenching of such austenite, smaller martensite plates are formed and stresses are reduced.
Thermo-mechanical processing is also used for other alloys.
Surface hardening of steel parts.
One of the methods of surface hardening of steel parts is surface hardening. As a result of surface hardening, the hardness of the surface layers of the product increases with a simultaneous increase in abrasion resistance and endurance limit.
Common to all types of surface hardening is heating the surface layer of the part to the hardening temperature followed by rapid cooling. These methods differ in the methods of heating the parts. The thickness of the hardened layer during surface hardening is determined by the heating depth.
The most widespread are electrothermal hardening with heating of products with high frequency currents (HFC) and gas-flame hardening with heating with a gas-oxygen or oxygen-kerosene flame.
Laser hardening is used to harden individual surfaces of parts, as well as parts of complex shapes, the performance of which is determined by wear resistance and fatigue strength, and surface hardening by other methods is difficult. The parts are heated with optical or gas (CO2) lasers. Optical lasers allow for pulsed heating, while gas lasers allow continuous heating. Continuous-wave lasers provide high productivity, uniform hardening and allow the processing of complex surfaces.
Cold processing of steel.
High-carbon and many alloy steels have an end-of-martensitic transformation temperature (Mc) below 0 °C. Therefore, in the structure of steel after hardening, a significant amount of retained austenite is observed, which reduces the hardness of the product and also worsens the magnetic characteristics. To eliminate residual austenite, additional cooling of the part is carried out in the region of negative temperatures, to temperatures below Mk (– 80 ° C). Dry ice is usually used for this. This treatment is called cold processing of steel. Cold treatment must be carried out immediately after quenching to prevent stabilization of austenite.
The increase in hardness after cold treatment is usually HRC 1 – 4. After cold treatment, the steel is subjected to low tempering, since cold treatment does not reduce internal stresses. Parts of ball bearings, precision mechanisms, and measuring instruments are subjected to cold treatment.
Strengthening by plastic deformation.
The main purpose of mechanical surface hardening methods is to increase fatigue strength. Methods of mechanical hardening - hardening of the surface layer to a depth of 0.2 - 0.4 mm. Varieties include shot blasting and roller finishing.
Test tasks
75. The structure, which is a solid solution of carbon in a-iron, is called:
a) perlite; b) cementite; c) ferrite; d) austenite.
76. The structure, which is a solid solution of carbon in γ-iron, is called:
a) cementite; b) ferrite; c) austenite; d) ledeburite.
77. The structure representing iron carbide Fe3C is called:
a) ferrite; b) austenite; c) ledeburite; d) cementite.
78. The structure, which is a mechanical mixture of ferrite and cementite, is called:
a) perlite; b) δ-ferrite; c) austenite; d) ledeburite.
79. The structure, which is a mechanical mixture of austenite and cementite, is called:
a) perlite; b) ferrite; c) ledeburite; d) δ-ferrite.
80. The eutectoid reaction occurs in the section of the iron–cementite diagram:
a) in the QPSKL area; b) in the SECFK area; c) on the ECF line; d) on the PSK line.
81. The eutectic reaction occurs in the section of the iron–cementite diagram:
a) on the ECF line; b) in the SECFK area; c) in the field of EIBC; d) on the PSK line.
82. The following process occurs on the HIB line of the iron–carbon diagram:
a) δ-ferrite crystals disappear; b) pearlite is formed; c) a peritectic reaction occurs; d) crystallization of hypoeutectoid steels is completed.
83. At room temperature, the structural component of iron-carbon alloys has the greatest plasticity:
a) austenite; b) ferrite; c) cementite; d) perlite.
84. The structural component of iron-carbon alloys has the greatest hardness:
a) austenite; b) perlite; c) ferrite; d) cementite.
85. Indicate the mass fraction of carbon in carbon hypereutectoid steel:
a) 0.02 < C < 0.8%; b) 4.3 < C < 6.67%; c) 2.14 < C < 4.3%; d) 0.8 < C < 2.14%.
86. Specify the structural composition of hypereutectoid steel at temperatures below 727 °C:
a) ledeburite + primary cementite; b) ferrite + tertiary cementite; c) perlite + secondary cementite; d) ferrite + pearlite.
87. The diagram (Fig. 5.17) shows the structure of steel:
| Rice. 5.17 |
a) technical hardware; b) eutectoid; c) hypereutectoid; d) hypoeutectoid.
88. The structural component marked with a question mark in the diagram of the structure of hypoeutectoid steel (Fig. 5.18) is called:
a) ferrite; b) austenite; c) secondary cementite; d) perlite.
| Rice. 5.18 |
89 .
Iron-carbon alloys are called cast iron: a) with a carbon fraction of more than 0.8%; b) with a carbon share of more than 4.3%; c) with a carbon share of more than 0.02%; d) with a carbon share of more than 2.14%.
90. Ledeburite is one of the structural components:
a) hypoeutectic white cast iron; b) steel at a temperature above the eutectoid transformation temperature; c) ferritic gray cast iron; d) technical hardware.
91. Indicate how the crystals that are being released at the moment differ from those that were released earlier during the equilibrium crystallization of an alloy of a system with a continuous series of solid solutions:
a) previously released crystals are richer in the refractory component; b) the composition of the crystals changes from component A to component B; c) there is no difference; d) previously released crystals are richer in the low-melting component.
92. Indicate how the crystals formed at this temperature differ from those formed earlier during nonequilibrium crystallization of an alloy of a system with a continuous series of solid solutions:
a) previously released crystals are richer in the refractory component; b) previously released crystals are richer in the low-melting component; c) during the crystallization process, the composition of the crystals changes from pure component A to component B; d) There is no difference.
93. The tendency (or lack thereof) of austenite grains to grow is called:
a) temper fragility; b) hereditary natural graininess; c) austenization; d) actual grain size.
94. Indicate which of the technological processes listed in the answers should be carried out taking into account the hereditary granularity:
a) cold pressure treatment; b) sand casting; c) high vacation; d) hardening, annealing.
95. Metallographic analysis of hereditarily fine-grained steel showed that its grain size is in the range of 0.05 - 0.08 mm. Please indicate which grain you mean:
a) real; b) initial; c) hereditary; d) original.
96. Indicate what explains the greater hardness of troostite than sorbitol:
a) the shape of cementite particles in troostite differs from the shape of particles in sorbitol; b) troostite has lower thermal stresses than sorbitol; c) troostite contains more (mass fraction) cementite particles than sorbitol; d) cementite particles in troostite are more dispersed than in sorbitol.
97. Martensite has a crystal lattice:
a) cubic; b) GPU; c) tetragonal; d) HCC.
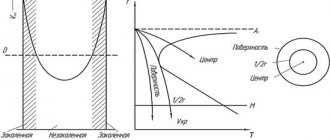
98. Indicate which of the cooling rates plotted on the isothermal decomposition diagram of austenite (Fig. 5.19) is critical:
| Rice. 5.19 |
a) V1;
b) V2; c) V3; d) V4. 99. A structure representing a supersaturated solid solution of carbon in α-iron is called:
a) martensite; b) cementite; c) ferrite; d) austenite.
100. The cooling rate during quenching is called critical:
a) the maximum cooling rate at which austenite still decomposes into pearlite-type structures; b) the minimum cooling rate required to obtain a martensitic structure; c) the minimum cooling rate required to fix the austenitic structure; d) the minimum cooling rate required to harden the product over its entire cross-section.
101. The main signs of martensitic transformation:
a) diffusion mechanism of transformation and a clear dependence of the transformation temperature on the cooling rate of the alloy; b) dependence of the completeness of transformation on the austenitization temperature and small distortions in the crystal lattice; c) weak dependence of the transformation temperature on the composition of the alloy and low stresses in the structure; d) diffusion-free transformation mechanism and oriented structure.
102. Indicate how the cooling rate during quenching affects the temperature at which the martensitic transformation begins:
a) the higher the cooling rate, the lower the temperature; b) the temperature at which the martensitic transformation begins does not depend on the cooling rate; c) the higher the cooling rate, the higher the temperature; d) the dependence of the temperature of the onset of martensitic transformation on the cooling rate is ambiguous.
103. The amount of retained austenite depends on:
a) on the temperature of the start and end points of the martensitic transformation; b) on the heating rate during austenitization; c) on the homogeneity of the original austenite; d) on the cooling rate of the alloy in the bending region of the C-shaped curves.
104. Critical points A3 of iron-carbon alloys correspond to the temperature values
a) 727 °C; b) 727 – 1147 °C (depending on the mass fraction of carbon); c) 727 – 911 °C (depending on the mass fraction of carbon); d) 1147 °C.
105. Point A from
3 means:
a) temperature point of the beginning of martensite decomposition; b) the temperature point at which the transformation of austenite into martensite begins; c) the temperature value of the critical point of transition of pearlite to austenite during nonequilibrium heating; d) the value of the critical point temperature, above which, during nonequilibrium heating, hypoeutectoid steels acquire an austenitic structure.
106. Critical points A m
located on the line of the Fe−C phase diagram:
a) PSK; b) SE; c) ECF; d) GS.
No. 107. Heat treatment of steel, consisting of heating it above A3 or A m
holding and subsequent rapid cooling is called:
a) true hardening; b )
full hardening;
c) incomplete hardening; d) normalization.
108. Hypoeutectoid steel after quenching at temperatures above A c
1, but below A
c
3 acquires the structural composition:
a) martensite + ferrite; b) perlite + secondary cementite;
c) martensite + secondary cementite; d) ferrite + pearlite.
109. Hardening of carbon hypereutectoid steels is carried out from the following temperature values:
a) from t
30 – 50 °C above At;
b) from t
30 – 50 °С below the line of the ECF diagram Fe – C;
c) from t
30 – 50 °C above the eutectic;
d) from
t
30
– 50 °C above A1.
110. Explain why incomplete hardening is not used for hypoeutectoid steels (unlike hypereutectoid steels):
a) martensite with a low degree of carbon supersaturation is formed; b) non-martensitic type structures are formed (sorbitol, troostite); c) the product is calcined to insufficient depth; d) ferrite inclusions remain in the structure along with martensite.
111. Temperature of steel hardening 50 (steel with a mass fraction of 0.5% carbon):
a) 600 – 620 °C; b) 810 – 830 °C; c) 740 – 760 °C; d) 1030 – 1050 °C.
112. Temperature of steel U12 hardening (steel with a mass fraction of 1.2% carbon):
a) 760 – 780 °C; b) 600 – 620 °C; c) 1030 – 1050 °C; d) 820 – 840 °C.
113. What is the mass fraction of carbon in the martensite of hardened steel grade 45 (steel with a mass fraction of 0.45% carbon):
a) 0.45%; b) 2.14%; c) 0.02%; d) 0.80%.
114. Hardening is:
a) penetration depth of the hardened zone; b) the process of formation of martensite; c) the ability of the metal to quickly warm up to its entire depth; d) the ability of a metal to increase hardness during hardening.
115 The difference between U10 and U12 steels (mass fraction of carbon 1.0 and 1.2%, respectively), hardened at a temperature of 760 °C:
a) in the structure of the U12 alloy there is more secondary cementite; b) there are no differences; c) in the martensite of the U12 alloy, the mass fraction of carbon is higher; d) the martensite of the U10 alloy is more dispersed than the U12 martensite.
116. Indicate how most alloying elements affect the martensitic transformation:
a) do not affect the transformation; b) shift the start and end points of the transformation to higher temperature values; c) shift the start and end points of the transformation to lower temperature values; d) narrow the temperature range of transformation.
117. Indicate the mass fraction of carbon in martensite of hardened steel grade U12 (with a mass fraction of carbon 1.2%):
a) ~ 0.02%; b) ~ 0.8%; c) ~ 2.14%; d) ~ 1.2%.
118. The critical diameter is called:
a) the diameter of the product, during hardening of which the critical hardening rate is ensured in the center; b) the maximum diameter of the product that accepts through hardening; c) the diameter of the product, during quenching of which a semi-martensitic structure is formed in the center; d) the maximum diameter of the product that is annealed through when cooled in a given quenching environment.
119. Indicate the dependence of the hardenability of steel on the cooling intensity during quenching:
a) the relationship between cooling intensity and hardenability is ambiguous; b) the more intense the cooling, the less hardenability; c) hardenability does not depend on the cooling intensity; d) the more intense the cooling, the greater the hardenability.
120. Arrange steel samples quenched in water, oil and air, according to the degree of decreasing depth of the hardened layer, if the sample quenched in water is not completely annealed:
a) in oil – in air – in water; b) in air – in oil – in water; c) in oil – in water – in air; d) in water – in oil – in air.
121. Through calcination provides:
a) increasing the hardness of the heat-treated product, however, the impact strength in the core is lower than in the outer layers; b) obtaining, after heat treatment, granular structures throughout the entire volume of the product and high mechanical properties uniform across the cross-section; c) obtaining the same hardness over the cross-section of the product; d) reduction in the amount of retained austenite, which leads to an increase in the mechanical properties of steel.
122. Explain the dependence of the hardness of the semi-martensitic structure of hypoeutectoid steel on the mass fraction of carbon:
a) the more carbon, the greater the hardness; b) the more carbon, the less hardness; c) the dependence is ambiguous. The hardness of the semi-martensitic structure is also determined by the nature of the heat treatment; d) hardness does not depend on the mass fraction of carbon.
123. Explain the effect of most alloying elements dissolved in austenite on the hardenability of steel:
a) increase hardenability; b) reduce hardenability; c) do not affect hardenability; d) the influence is ambiguous. There is a high dependence on vacation schedules.
124. Explain which alloy has a larger critical diameter if alloy A has a higher critical hardening rate than alloy B:
a) for alloy A; b) for alloy B; c) the relationship between the critical hardening rate and the critical diameter is ambiguous; d) the critical diameter does not depend on the critical quenching rate.
125. Through hardenability of large parts is achieved:
a) repeated hardening; b) the use of high-speed coolers during hardening; c) treatment after cold hardening; d) use for the manufacture of alloy steel parts.
126. Heat treatment, consisting of heating hardened steel below A1, holding and subsequent cooling, is called:
a) annealing; b) austenization; c) vacation; d) normalization.
127. The hardened product acquires the greatest ductility:
a) with low holidays; b) during high holidays; c) does not depend on the type of leave; d) during an average vacation.
128. Indicate what type of heat treatment of hypoeutectoid steels produces granular structures:
a) during isothermal hardening; b) when hardening at a speed above critical; c) with complete annealing; d) when given for sorbitol or troostitis.
129. Explain how heating temperature during tempering affects the hardness of carbon steel products:
a) the effect of tempering temperature on hardness is ambiguous; b) the higher the heating temperature, the higher the hardness; c) the higher the heating temperature, the lower the hardness; d) hardness does not depend on the tempering temperature.
130. Explain under which heat treatment of carbon steel the formation of a granular sorbitol structure is most likely:
a) during normalization; b) with improvement; c) during martensite quenching and medium tempering; d) when hardened with sorbitol.
131. Heat treatment consisting of hardening and high tempering is called:
a) normalization; b) improvement; c) spheroidization; d) complete hardening.
132. Explain how most alloying elements affect transformations in steel during tempering:
a) inhibits the process of martensite-pearlite transformation, shifting it to the region of higher temperature values; b) does not affect transformations during tempering; c) shifts the process of martensite-pearlite transformation to the region of lower temperature values; d) accelerates the martensite-pearlite transformation.
133. Treatment consisting of long-term exposure of a hardened alloy at room temperature or at low heat is called:
a) recrystallization; b) normalization; c) high holidays; d) aging.
134 . Heat treatment of steel, consisting of heating it above A3 or A m,
holding and subsequent cooling together with the furnace is called:
a) incomplete annealing; b) complete annealing; c) recrystallization annealing; d) low annealing.
135. Indicate which annealing should be used to remove strain hardening:
a) recrystallization; b) complete (phase recrystallization); c) spheroidizing; d) diffusion.
136. Purpose of diffusion annealing:
a) homogenization of the structure; b) stress relief in the crystal lattice; c) improvement of the ferrite component of the structure; d) obtaining a granular structure.
137. Explain how the depth of the hardened layer is adjusted when heated by high-frequency currents:
a) current strength; b) cooling intensity; c) current frequency; d) type of coolant.
138. The heat treatment of steel, which consists of heating it to an austenitic state and subsequent cooling in still air, is called;
a) true hardening; b) improvement; c) incomplete annealing; d) normalization.
139. Indicate what features the state diagram of the saturable metal – saturating component system should have to carry out chemical-thermal treatment:
a) CHT is possible only for systems that form mechanical mixtures of component crystals; b) there must be a high-temperature region of significant solubility of the component in the metal; c) CHT is possible only for systems that form continuous solid solutions; d) the diagram must contain stable chemical compounds.
140. The ultimate goal of steel carburization is:
a) creating a fine-grained core structure; b) increasing the mass fraction of carbon in steel; c) obtaining a solid surface layer in the product while maintaining a viscous core; d) increasing the plasticity of the surface layer.
141. A carburizer is:
a) a substance that serves as a source of carbon during cementation; b) carbides of alloying elements; c) a device for obtaining a fuel-air medium; d) a mixture of carbon dioxide salts.
142. The structure of the diffusion layer obtained as a result of steel carburization, starting from the surface, follows the following structures:
a) cementite + perlite; perlite; pearlite + ferrite;
b) cementite + ferrite; perlite; ferrite;
c) pearlite + ferrite; ferrite; ferrite + cementite;
d) perlite; perlite + cementite; cementite + ferrite.
143. The difference between martensite obtained after hardening a cemented product in the core areas and martensite in the outer layers:
a) pearlite-type structures are formed in the core due to the low hardenability of steels; b) in the outer layers there is high-carbon martensite, in the core it is low-carbon; c) there is no martensite in the core; d) in the outer layers the martensite is finely needle-shaped, in the core it is coarsely needle-shaped.
144. A treatment consisting of saturating the steel surface with nitrogen and carbon in molten salts with the CN group is called:
a) nitrocarburization; b) improvement; c) cyanidation; d) modification.
145. Treatment consisting of saturating the steel surface with nitrogen and carbon in a gaseous environment is called:
a) cyanidation; b) improvement; c) modification;
d) nitrocarburization.
146. Steels subjected to cementation:
a) high-carbon (more than 0.7% C); b) highly alloyed; c) low carbon (0.1 – 0.25% C); d) medium-carbon (0.3 - 0.5% C).
147. Heat treatment, which consists of heating, holding and subsequent slow cooling of the workpiece together with the furnace, is called:
a) vacation; b) rest; c) annealing; d) normalization.
Technology of cold hardening and cold hardening of metal
Hardening is a phenomenon in which the strength and hardness of a metal product increases. Changes in properties are achieved through plastic deformation. Metal hardening occurs at a high temperature, the value of which is not sufficient for recrystallization of the workpiece. This phenomenon can be both harmful and beneficial.
Hardening is a technological process that pursues the same goals as cold hardening. The main difference is that the latter phenomenon can occur as a result of conscious or unconscious actions.
For example, during machining by cutting with high speed and cutting depth, the surface acquires excess strength, which increases the fragility of the product. Cold hardening is only useful strain hardening, the use of which is deliberate.
The essence and purpose of cold hardening and cold hardening
As a result of plastic deformation, changes occur in the crystal lattice and phase composition of the material. The process of cold hardening of metal is accompanied by the formation of defects in the internal structure of the product. In this case, the properties of the material change as follows:
- resistance to mechanical damage increases (metal strengthening);
- the hardness of the material increases;
- resistance to dynamic loads is reduced;
- plasticity is lost;
- there is a decrease in resistance to plastic deformation with the opposite sign - this is called the Bauschinger effect.
Thus, the yield strength of the metal is reduced. This parameter determines the maximum stress on the product at which it will begin to deform plastically. If the degree of load does not exceed the permissible value, after the cessation of external forces, the metal will return to its previous state.
This parameter is especially important for cold-worked steel, which is used as the main material in load-bearing structures of various buildings and structures. The project is drawn up taking into account the maximum loads on individual elements and the object as a whole.
The study of the metal structure suggests that after exceeding the yield strength, the product receives strain hardening. To harden the surface by cold hardening, special equipment is used, which will be discussed below.
When exposed to steel and other ferromagnetic materials, an increase in the magnetic field strength is observed. This parameter is called coercive force. At the same time, the magnetic permeability of the product decreases.
The phenomenon under consideration helps to improve the performance properties of ductile metals. When cold-working aluminum and alloys based on it, a significant increase in hardness and increase in yield strength are observed.
The convenience of working with ductile metals lies in the fact that for cold deformation processing you can use any of the following methods:
- rolling;
- deep drawing;
- forging;
- flange.
In what cases is cold hardening used, and when is cold hardening used?
The physics of these processes is based on strain hardening of a metal product. The difference is this:
- Hardening is any strain hardening of metal, the effect of which can be both positive and negative.
- Hardening is considered only a process that is deliberately applied to a product in order to improve performance characteristics.
Technical documentation, including state standards, ANSI and ISO, does not contain the term peening. For example, deformation-hardened aluminum is called cold-worked aluminum. For this metal, the degree of processing is indicated by the letter H. It is followed by a numerical definition, which can contain from one to three digits.
Types of hardening
Strain hardening of metal is classified according to the processes that are activated in the workpiece during the formation of the work-hardened layer.
In the case of the formation of new phases that differ in a different specific volume, the phenomenon is called phase. If the cause of the changes is the action of external forces, hardening is called deformation hardening.
There are two categories:
- Centrifugal ball. The product is affected by balls that are located in the rim sockets of the installation. Its operating principle is based on rotation, when, under the influence of centrifugal force, the elements exert a mechanical effect on the workpiece being processed.
- Shot blaster. This method is based on the use of kinetic energy. Shots with a diameter of up to 4 mm, made of durable material: cast iron, steel or ceramics, are used as processing elements. According to technological requirements, the flow speed can reach 70 m/s.
Technology of hardening and hardening of metal, tape, in Russia | MetalEnergoHolding
In the metal rolling industry, cold hardening or strain hardening is a controlled technological process that is used to increase the hardness of metals and increase their strength characteristics.
This technology is applied to those materials that cannot be refined by heat treatment. Hardening is not used to change the mechanical properties of rolled products made of copper, aluminum alloys, low-carbon steels, and chromium-nickel alloys.
For such materials, strain hardening is the only way to increase strength characteristics.
The definitions cold hardening and cold hardening are used to denote the process of changing the structure of the metal, as well as increasing its hardness as a result of external influence. At the same time, the concept of hardening includes both natural processes occurring in the structure of the metal and those controlled by special processing methods.
According to its origin, cold hardening can be:
- Phase. In this case, structural changes are caused by phase changes that occur as a result of heat treatment of the metal.
- Deformation. Strengthening and increasing hardness occurs as a result of the influence of external forces.
In particular, phase hardening (undesirable) occurs when cutting alloys that have ductility and softness. Too deep a cut with a large thickness of the workpiece, made at high speed, causes intense hardening, a decrease in the ductility of the metal, and an increase in fragility.
Unlike cold hardening, cold hardening is a controlled process. Hardening is not always beneficial. When hardening occurs, the plastic properties of materials decrease. For example, the ductility of low-carbon steel alloys is reduced by more than 5 times. At the same time, there is a decrease in the resistance of the metal to mechanical stress - tensile, tensile, compressive and bending loads.
Cold-frettening of strain-hardening alloys
Structure modification
These alloys include all alloys of the 1xxx, 3xxx and 5xxx series, as well as some alloys of the 8xxx series. Their process chain consists of hot working steps, possibly followed by cold working steps with intermediate or final annealing.
Strain hardening - cold hardening - involves modification of the structure under the influence of plastic deformation. This occurs not only during the production of semi-finished products during rolling, straightening, stretching, drawing, etc., but also during subsequent production steps such as forming, bending and other production operations.
Mechanical properties
Strain hardening increases mechanical strength properties and hardness, but reduces ductility (Figure 6).
The level of mechanical properties that can be achieved depends on the alloying elements. For example, alloys of the 5xxx series, which contain large amounts of magnesium, have a higher potential level of mechanical properties than alloys of other series: 1xxx, 3xxx and 8xxx. The result is always a gradual increase in mechanical properties, up to a point where further processing becomes difficult, if not impossible. In this case, if further plastic deformation is required, it is necessary to carry out annealing heat treatment.
Softening annealing
Hardening that occurs as a result of cold plastic working can be eliminated or softened by annealing. Depending on the duration-temperature combination, this softening can be (Figure 7):
- partial: this is softening or incomplete annealing;
- complete: this is recrystallization annealing, during which a new grain structure is formed (Figure (8).
Figure 7 – Isothermal annealing curves of alloy 5754
Figure 8 – Change in hardness and structure during annealing
Time and temperature parameters are specific to each alloy and depend on the degree of strain hardening to which the material was subjected before annealing.
Like other metals and alloys, there is a critical zone of strain hardening (Figure 9.1). If annealing is applied to a material in a state that is in this critical zone, uncontrolled grain growth may occur. This makes subsequent forming operations such as drawing and bending more difficult. After deformation, the metal surface may have an appearance called “orange peel”.
Figure 9.1 – Change in grain size during annealing depending on the degree of cold hardening
The level of mechanical properties of the semi-finished product and, in particular, the compromise between tensile strength and ductility (elongation), are controlled by the parameters of deformation processing and subsequent annealing operations (intermediate or final).
It should be noted that at the same level of tensile strength, the level of ductility will be higher in cold-worked and partially annealed metal (H2X) than in “purely” cold-worked metal (H1X) (Figure 9.2). Partial (softening) annealed conditions are therefore preferred when maximum formability is a major factor, such as in deep drawing.