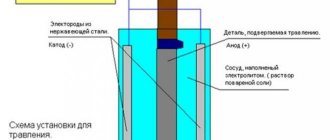
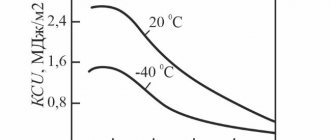
History[edit]
Ferritic stainless steels were discovered early, but it was not until the 1980s that conditions were created to grow them:
- Very low levels of carbon could be produced during the steelmaking stage.
- Weldable grades have been developed.
- Using electromechanical machining, the problems of ropes and protrusions that caused uneven deformation during deep drawing and textured surfaces were resolved.
- End-use markets (such as the white goods market) demanded less expensive grades with a more stable price at a time when nickel prices fluctuated widely. [3] Ferritic grades of stainless steel are attractive for use in some applications, such as cookware. [4]
Austenitic stainless steels
Nickel is an element that increases the stability of austenite. The presence of nickel in steel increases the size of the austenitic region, while ferrite almost completely disappears from iron-chromium-carbon alloys (Figure 3).
Figure 3 – Cross-section of the iron-chromium-nickel-carbon phase diagram at 18% chromium and 8% nickel. At low carbon content, austenite is stable at room temperature.
If the carbon content drops below 0.03%, then carbides are not formed in the steel at all and the steel is completely austenitic at room temperature (Figure 4).
Figure 4 – Austenitic stainless steel
Austenitic stainless steels have high ductility, pressure machinability, and corrosion resistance.
Heat treatment of austenitic stainless steels involves quenching in water from a temperature of 1050-1100 °C. Such heating causes the dissolution of chromium carbides, and rapid cooling fixes the state of a saturated solid solution. It is very important to note that as a result of hardening, the hardness of these steels does not increase, but decreases. Therefore, for austenitic stainless steels, hardening is a softening thermal operation.
Austenitic stainless steel gains its strength through cold hardening. Austenitic steels can be strain hardened to significantly higher values than ferritic stainless steels. At deformations of the order of 80-90%, the yield strength reaches 980-1170 MPa, and the tensile strength reaches 1170-1370 MPa. It is clear that such hardening can only be achieved in the manufacture of such types of products as thin sheets, tape, and wire.
Austenitic stainless steels are non-magnetic, which gives them an advantage in many applications.
Representatives of austenitic stainless steels are steels 12Х18Н9 and 17Х18Н9, 12Х18Н10Т and 12Х18Н9Т, 08Х18Н10Т, 08Х18Н12Б, 03Х18Н11 according to GOST 5632-72.
Metallurgy[edit]
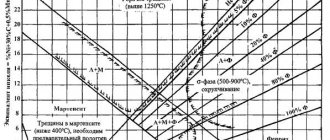
Fe - Cr Phase Diagram
To be considered stainless steel, Fe-based alloys must contain at least 10.5% Cr.
The iron-chromium phase diagram shows that up to about 13% Cr, the steel undergoes successive transformations upon cooling from a liquid phase from a ferritic α phase to an austenitic γ phase and back to α. When some carbon is present and if cooling occurs rapidly, some of the austenite will change to martensite. Quenching/annealing converts the martensitic structure into ferrite and carbides.
With a Cr content above about 17%, the steel will have a ferritic structure at all temperatures.
Above 25% Cr, the sigma phase can develop over a relatively long time at temperature and cause embrittlement at room temperature.
Martensite and its structure
The basis of martensitic steel is martensite. This is a special way of distributing molecules: the metal molecules are arranged in such a way that they form a needle-like structure. Martensite appears and is fixed in alloys that have been quenched, as well as in metals prone to polymorphism. This structure is formed during the cooling process after hardening. If you look at it, you can see a tetragonal crystal lattice of a carbon solution in alpha iron. Thanks to martensite, martensitic steel acquires amazing strength and hardness - these are very important properties for industry. Martensite is a nonequilibrium structure, due to which it forms internal stresses. If martensitic steel is heated, the carbon atoms in its structure will redistribute. As a result of this redistribution, two phases will form - cementite and ferrite.
Chemical composition of several grades (main alloying elements) [edit]
Chemical composition (balance: Fe)
| AISI/ASTM | EN | Weight % | |
| Cr | Other items | ||
| 405 | 1,4000 | 12,0 — 14,0 | — |
| 409L | 1,4512 | 10,5 — 12,5 | 6 (C+N) <0.65 |
| 410L | 1,4003 | 10,5 — 12,5 | 0,3 <1,0 |
| 430 | 1,4016 | 16,0 — 18,0 | — |
| 439 | 1,4510 | 16,0 — 18,0 | 0.15 + 4 (C + N) <0.8 |
| 430Ti | 1,4511 | 16,0 -18,0 | Ti: 0.6 |
| 441 | 1,4509 | 17,5 — 18,5 | 0,1 <0,6 0.3 + 3C <1.0 |
| 434 | 1,4113 | 16,0 — 18,0 | 0.9 <Mo <1.4 |
| 436 | 1,4513 | 16,0 — 18,0 | 0.9 <Mo <1.4 0,3 <0,6 |
| 444 | 1,4521 | 17,0 — 20,0 | 1.8 <Mo <2.5 0.15 + 4 (C + N) <0.8 |
| 447 | 1,4592 | 28 — 30,0 | 3.5 <Mon <4.5 0.15 + 4 (C + N) <0.8 |
Dual phase stainless steels
In some cases, a mixture of different phases is deliberately obtained in the structure of stainless steels. With appropriate control of the chemical composition and heat treatment conditions, steel is obtained containing, for example, 50% ferrite and 50% austenite. This combination of phases in the structure of the steel gives it a unique combination of mechanical properties, corrosion resistance, workability and weldability that cannot be achieved in any other stainless steels. Sometimes they are called in foreign languages - duplex steels.
Two-phase stainless steels include steels 08Х22Н6Т, 03Х23Н6, 08Х21Н6М2Т, 03Х22Н6М2, 08Х18Г8Н2Т, 03Х24Н6М3 according to GOST 5632-72.
Source: D. Askeland, P. Fulay, W. Wright – The Science and Engineering of Materials, 2011
Corrosion resistance[edit]
The resistance to pitting corrosion of stainless steels is assessed by the equivalent PREN number.
PREN =% Cr + 3.3% Mo + 16% N, where the terms correspond to the content of chromium, molybdenum and nitrogen by weight in the steel, respectively.
Nickel plays no role in resistance to pitting corrosion, so ferritic stainless steels can be as resistant to this form of corrosion as austentitic grades.
In addition, ferritic grades are very resistant to stress corrosion cracking (SCC).
Where are alloys used?
The martensitic class of steel, due to its special structure, has a number of excellent characteristics. For example, such alloys are characterized by high strength and resistance to deformation. Therefore, martensitic steel is used for the production of power equipment.
Resistant to high temperatures and oxidation, this steel is ideal for the manufacture of engine parts, compressor valve vanes, rotors and turbines. The alloy has also found application in medicine—cutting instruments are made from it. Martensitic steel is generally not used in corrosive environments because the material is not resistant to MCC.
Physical properties[edit]
Ferritic stainless steels are magnetic
Physical properties of the most common ferritic stainless steels
| AISI/ASTM | Density g/cm3 | Electrical Resistance µOhm.m | Thermal Electrical conductivity at 20°C W/(m °K) | Specific heat 0 - 100 ° C J/(kg.°K) | Thematic extension 0 - 600 ° C 10−6/°K | Module for junior GPA |
| 409/410 | 7,7 | 0,58 | 25 | 460 | 12 | 220 |
| 430 | 7,7 | 0,60 | 25 | 460 | 11,5 | 220 |
| 430Ti/439/441 | 7,7 | 0,60 | 25 | 460 | 11,5 | 220 |
| 434/436/444 | 7,7 | 0,60 | 23 | 460 | 11,5 | 220 |
| 447 | 7,7 | 0,62 | 17 | 460 | 11 | 220 |
Compared to austenitic stainless steel, they have better thermal conductivity, which is a plus for applications such as heat exchangers.
A coefficient of thermal expansion close to that of carbon steel makes carbon steels easier to weld.
Ferritic stainless steels
Ferritic stainless steels contain up to 30% chromium and no more than 0.12% carbon. Due to their body-centered crystal structure (BCC), ferritic steels have good strength and decent ductility, which are achieved through solid solution strengthening and strain hardening. Ferritic steels are ferromagnetic or, in simple terms, “magnetic”. They are not amenable to heat treatment. Ferritic steels have excellent corrosion resistance, moderate workability, and are relatively inexpensive.
Ferritic stainless steels include steels 08Х13, 12Х17, 08Х17Т, 15Х25Т, 15Х28 according to GOST 5632-72.
Mechanical properties[edit]
Mechanical properties (cold rolled)
| ASTM A240 | EN 10088-2 | ||||||
| UTS MPa, min | 0.2% yield Stress MPa, min | Elongation %, min | UTS MPa | 0.2% yield Stress MPa, min | Elongation %, min | ||
| 409 | 390 | 170 | 20 | 1,4512 | 380–560 | 220 | 25 |
| 410 | 415 | 205 | 20 | 1,4003 | 450 — 650 | 320 | 20 |
| 430 | 450 | 205 | 22 | 1,4016 | 450–600 | 280 | 18 |
| 439 | 415 | 205 | 22 | 1,4510 | 420–600 | 240 | 23 |
| 441 | 415 | 205 | 22 | 1,4509 | 430–630 | 250 | 18 |
| 434 | 450 | 240 | 22 | 1,4113 | 450–630 | 280 | 18 |
| 436 | 450 | 240 | 22 | 1,4526 | 480-560 | 300 | 25 |
| 444 | 415 | 275 | 20 | 1,4521 | 420–640 | 320 | 20 |
Precipitation hardening stainless steels
These steels are also called high-strength stainless steels. Precipitation hardening stainless steels contain aluminum, niobium or tantalum and obtain their properties through quenching, strain hardening, age hardening and martensitic transformation. The steel is first heated and hardened to transform austenite into martensite. Reheating causes the precipitation of strengthening particles such as NiAl3 from the martensite. The high strength of these steels is achieved even with low carbon content.
Dispersion-hardening steels include steels 07Х16Н6, 09Х15Н8У, 08Х17Н5М3, 04Х25Н5М2, ХН40МДТУ according to GOST 5632-72.
Welding methods and welding materials
The presence of active alloying elements Cr, Ti, AI in steels determines the use of welding methods and welding materials that limit the loss of alloying elements: electrodes with a basic or fluoride type of coating, inert Ar and He or weakly oxidizing mixtures of inert and active gases Ar + 1 - 3% O2 and Ar + 2 - 4% CO2, passive fluoride and basic fluoride, low-active and active low-silicon fluxes, depending on alloying.
Ferritic steels
1. Manual arc welding with ferrite electrodes, which produces weld metal of the same or similar chemical composition to the base metal.
2. Arc welding in inert gases with a non-consumable tungsten electrode and consumable ferritic and, less commonly, austenitic wires.
3. Automatic submerged arc welding with ferritic and less commonly austenitic wires using basic, weakly oxidizing low-silicon and basic-fluoride fluxes.
Austenitic steels
1. Manual arc welding with austenitic electrodes, giving a weld metal composition that is resistant to hot cracks (usually austenite + 2 - 10% ferrite) and pores caused by hydrogen.
2. Arc welding in inert gases with non-consumable and consumable electrodes with an austenitic filler, which should also ensure the resistance of the seam against hot cracks, pores and ICC. When welding thin sheet metal, it is recommended to use a mixture of Ar + 3% O2 or Ar + 15 - 20% CO2 to reduce the critical current, improve formation and prevent porosity. CO2 and N2 can be used as shielding gases when welding certain grades of austenitic steels.
3. Automatic submerged arc welding is usually performed using electrode wires of the same type as gas-shielded welding. Fluxes use low-silicon basic and basic fluoride fluxes.
Arc welding, as a rule, should be performed at limited heat input, with small cross-section beads, to avoid the formation of a coarse dendritic structure in the weld, grain growth in the HAZ and hot cracks.
Groups of stainless steels by chemical composition
Depending on the set of main alloying elements in the chemical composition, the following groups of stainless steels are distinguished:
- Chromium.
- Chrome-nickel.
- Chromium-manganese-nickel.
Chrome steels
As is clear from the name of the group, the main alloying element of chromium steels is chromium. According to GOST 5632-2014, the nominal chromium content can be 13, 17 or 25/28%. The first type includes brands 08Х13, 13х13, 20Х13, 30Х13, 40Х13, the second - 12Х17 and 08Х17Т, the third - 15Х25Т and 15Х28. Chromium steels of the second and third types belong to the ferritic class, and the first type can have a ferritic, martensitic or ferritic-martensitic class.
Chrome-nickel steels
Chrome-nickel steels contain 14-20% chromium, 12-14% nickel. They are resistant to acids and high temperatures, and are well suited to technological deformation, in particular, stamping and welding. They can be processed satisfactorily by cutting. Chromium-nickel steels include steel grades 20X17N2, 14X17N2, 20X17N2, 14X17N2.
Chromium-manganese-nickel steels
Partial replacement of nickel with cheaper manganese helps reduce the cost of the material without noticeably reducing its useful properties. The addition of manganese increases the ductility of stainless steel and helps maintain non-magnetic properties, increasing impact strength at low temperatures. But, it should be taken into account that chromium-manganese-nickel steels are difficult to weld and are prone to temper brittleness. The main representatives of this group: 03X20N16AG6, 07X21G7AN5, 10X14G14N4T.
Taking into account the structure of their crystal lattice, chromium-nickel and chromium-manganese-nickel steels are divided into austenitic, austenitic-ferritic, austenitic-ferritic and austenitic-carbide.