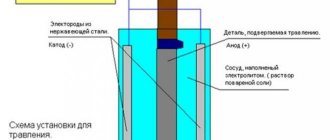
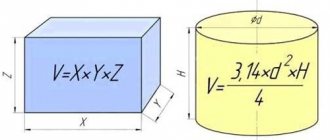
Corrosion
Many malfunctions of equipment elements intended for the synthesis, storage and transportation of aggressive substances are observed as a result of local corrosion. The most common types of corrosion are intergranular corrosion, knife corrosion, and stress corrosion. Let's briefly look at these types. Corrosion of equipment in nitric acid solutions.
Intergranular corrosion (ICC). Known cases of MCC in operating conditions of equipment made of steel 12Х18Н9Т can be divided into the following groups:
- destruction of the metal over large areas of the surface and in depth (the tendency of the metal to MCC was discovered as a result of deviation from the heat treatment regime); destruction of the metal under the surface, while the surface of the metal has a network of cracks visible upon magnification (areas of repeated heating);
- destruction of metal to a small depth (0.1-0.3 mm) over the entire surface of metal structures of tanks and pipelines in contact with the steam-air phase of nitric acid (the surface layer of the metal had a changed structure of deformation, bending, hardening, etc.);
- local destruction of metal near welds as a result of changes in the structure of the metal associated with deviation from the welding mode;
- destruction of metal in cracks and gaps, defects in welds, etc. (crevice corrosion) as a result of structural heterogeneity of the weld metal.
A sharp decrease in the corrosion resistance of metal occurs as a result of the influence of technological factors during the manufacture and repair of equipment associated with changes in structure, metal contamination, the presence of residual stresses, etc.
There are a number of hypotheses for the appearance of the tendency of austenitic steels to MCC. The basis of each of them is the fact of the formation of new phases along grain boundaries and, above all, metal carbides. The latter depends on the solubility of carbon in the alloy. The destruction of the metal can be facilitated by the released phase, which is not sufficiently stable in this aggressive environment. A tendency to MCC occurs during the repair of pipeline structures during gas cutting (removal of defective sections). In the melt zone, titanium carbides decompose into carbon and titanium and are evenly distributed throughout the volume of the metal.
Under rapid cooling conditions, an austenitic structure is formed. In the case of slow cooling or reheating (up to 650°C), free carbon diffuses to the grain boundaries. Chromium binds into carbides, and the corrosion resistance of the boundary areas is reduced.
A decrease in the corrosion resistance of austenitic steels also occurs when the sigma phase precipitates in the temperature range 600–900° C. The temperature and inhomogeneity of the steel in chromium content have the greatest influence on the rate of formation of the sigma phase. It is known that titanium reduces the temperature of formation of the sigma phase to 430° C. The presence of the sigma phase in combination with MCC causes a rapid loss of mechanical strength of parts and even through destruction.
Intergranular corrosion. Process mechanism
The mechanism of the process of intergranular corrosion under conditions of exposure to oxidizing environments such as nitric acid can be represented as follows. As a result of unfavorable heat treatment or welding conditions, grain boundaries become depleted of chromium.
The diffusion of carbon from the solid solution to the grain boundaries proceeds much faster than the diffusion of chromium. Carbon diffusion occurs from the entire grain mass, while chromium comes only from the boundary zones of austenite. The chromium content in these zones drops so much that the zone loses its ability to passivate and undergoes rapid destruction in oxidizing environments.
The destruction of low-resistant phases depleted in chromium leads to the accumulation of corrosion products with a high iron content, which autocatalytically accelerate the dissolution of grain boundaries. In places where the secondary phase separates and gradually grows (at the boundaries of differently oriented grains), high local stresses appear. Significant energy differences arise, which can manifest themselves with a decrease in anodic polarization in the boundary zones between grains, as well as insufficient passivation of grain boundaries.
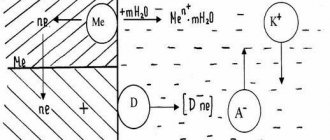
Finally, MCC as a particular type of electrochemical corrosion is the result of the work of microelements. The metal carbide acts as the cathode and the less noble metal surrounding it acts as the anode. If we take into account the fact that the boundary areas are depleted of chromium, it becomes clear why the anodic reaction does not spread over a large area (grain surface). Initially, due to the presence of defects (cracks, pores) in the initial oxide film, the process of metal destruction predominantly proceeds through the mechanism of uniform corrosion. The resulting poorly soluble corrosion products “heal” defects in the oxide film, reducing the area of the anodic areas. In the subsequent period, the role of the most effective anodes is played by the boundary areas of grains with a reduced chromium content, and the destruction of the metal further occurs through the mechanism of structural corrosion.
The development process of ICC can be divided into several stages. In the first stage, the rates of uniform corrosion and MCC are equal, and they cannot be distinguished by external signs. In the second stage, the rate of MCC exceeds the rate of uniform corrosion, but corrosion still occurs without visible destruction of the metal (incubation period). The third stage is characterized by the loss of single grains. At the fourth stage, metal destruction occurs with group loss of grains and loss of its mechanical strength.
Sometimes the last two stages occur inside the metal (subsurface corrosion). This division can be justified on the basis of electrochemical and diffusion concepts about the MCC process. Initially, the development of MCC occurs at a low speed due to diffusion restrictions. Corrosion products form in intercrystalline cracks, filling the cracks and making it difficult for the electrolyte to access. In addition, the distance between microanodes and microcathodes increases. In the case of low aggressiveness of environments, the increasing depth of damage and the density of corrosion products, together with the increasing separation of the cathode-anode sections over time, can lead to a logarithmic law of development of the ICC (for example, in a weak aqueous solution of nitric acid in the absence of stress in the metal). In more aggressive environments, intensive dissolution of corrosion products between grains occurs, and the environment freely reaches the destruction front. The anodic and cathodic sections come closer together, and the corrosion rate increases to 3-4 mm per year.
Knife corrosion.
Knife corrosion, like crevice corrosion, is a type of local corrosion, but unlike crevice corrosion, it is characteristic only of welded joints.
The causes of knife corrosion have not yet been fully revealed. It is considered either as a special case of the MCC of the base metal, concentrated in a narrow heat-affected zone, the rest of the metal is characterized by high corrosion resistance and remains in a passive state, or it is associated with the presence of the a-phase at the site of fusion of the base metal with the weld metal.
An increase in the inhomogeneity of austenite occurs as a result of heating steels above 1300 ° C and the subsequent precipitation of secondary phases along grain boundaries in the interface between the deposited and the base metal. It has been established that the width of the chromium-depleted zone is 2–3 µm. The chromium content in it is less than 10%. In the electrolyte, this zone exhibits an anodic character. Along the grain boundaries in a narrow overheated zone adjacent to the weld, a network of dendritic precipitates of titanium carbides is also formed.
Overheating of steel leads to grain growth, while their total surface area decreases and the thickness of chromium carbides at the grain boundaries increases. This mechanism is confirmed by the fact that subsequent low-temperature hardening (annealing) of welds helps prevent knife corrosion. The development of knife corrosion is possible by two mechanisms, depending on the value of the total stationary potential, determined by the value of the redox potential of the environment. At a low stationary potential, the base metal and precipitated chromium carbides are in a passive state, and the zone with a depleted solid solution is in an active state, as a result of which the effect of knife corrosion is detected. At a high stationary potential (repassivation conditions), the entire narrow zone of the base metal adjacent to the fusion line is in the active state.
In this case, chromium carbides will also be destroyed. Regardless of the interpretation of the mechanism of knife corrosion, the fact of destruction of a narrow zone immediately adjacent to the weld metal, which differs from the base metal in micro- and macrostructure, chemical composition, electrode potential and stress state, is noted.
Underground corrosion
Underground corrosion of metal and concrete structures is a process of their destruction that occurs through an electrochemical mechanism when exposed to ground moisture. The latter is an electrolyte of varying acidity (alkalinity). Uneven wetting of the metal with the ground electrolyte causes the formation of corrosive elements (steams).
The corrosive activity of soils is determined by: soil type; composition and concentration of salts contained in it; moisture content; pH environments; aeration and soil structure; temperature and soil resistivity; loss of metal mass; the presence of microorganisms in the soil, etc.
There are several types of soils. There are clayey and dusty soils (clays, suspensions, loams, forests). Clastic (pebbles, crushed stones, gravelly soils and sands), peaty, artificial and bulk.
The size of soil particles and the relationship between them and soil moisture play an important role. The danger in terms of corrosion is represented by soils whose particles have a physical and mechanical form of connection with moisture.
High water saturation of the soil in contact with reinforced concrete structures, under unfavorable conditions, leads after a few years to corrosion exceeding permissible limits (1.5-3 mm/year). Such conditions are increased soil acidity to moisture, the presence of direct contact of moisture with metal structures, etc.
Biological corrosion
Processes associated with the life of microorganisms are classified as biochemical processes. They can affect the corrosion resistance of metals and the protective resistance of coatings, contributing to the occurrence or acceleration of corrosion destruction.
Biocorrosion is the process of corrosive destruction of metal under the influence of microorganisms (bacteria, actinomycetes, filamentous fungi) along with other environmental factors.
According to a number of experts, about 50% of cases of corrosion are associated with the activity of microorganisms. The first mention of the destruction of metal elements of a water supply network by plant debris dates back to 1886. The destruction of a metal pipeline in the soil in 1910 was explained as a consequence of the activity of bacteria.
The number of studies of biological corrosion increased significantly after the Second World War, when military equipment and weapons transported to tropical countries as a result of sea transportation and storage in warehouses turned out to be unsuitable for combat operations. The cause of destruction was corrosion , aggravated by the influence of microorganisms. A more thorough study of microbiological damage to materials began 25-30 years ago, when the problem of protecting metal structures used in tropical countries became especially acute.
Only taken into account biogenic losses annually amount to over 2% of the cost of produced materials. In the USA, these losses are estimated at 1.5 billion dollars per year. According to Japanese experts, almost all electrical equipment, optical instruments, etc. in the warm, humid climate of Southeast Asia are affected by filamentous fungi. Tests of electronic industry products for fungal resistance have shown that most materials (up to 60%) have insufficient biological resistance. We observed the destruction of fuel tanks and aircraft communications by bacteria, and the clogging of filters with the products of their vital activity.
The above facts indicate that the impact of microorganisms on structural elements can lead to equipment failures during operation.
Fighting intergranular corrosion [edit | edit code ]
The resistance of the material to this type of corrosion can be increased by the correct choice of heat treatment modes, reducing the impurity content, and alloying with elements that prevent the formation of unwanted excess phases along grain boundaries, for example, titanium, niobium, tantalum, which form more stable compounds with carbon than chromium carbide.
A good preventative measure is to reduce the carbon content in the main part, and in welding and in the welding material, to a level of less than 0.02%.
It is possible to heat the product up to 1000 °C and quench it in water, which leads to the dissolution of carbides in the grains and prevents their re-isolation.
When welding sufficiently thin layers of material, the material does not have time to warm up to temperatures leading to intergranular corrosion.