29Jan
- By: Semantics
- Uncategorized
- Comments: 0
Depending on the purpose of the material and specific operating conditions, there are different methods for manufacturing steel elements.
In the article we will tell you what the process of alloying metals and steels is, for what purpose it is carried out, and what is used for the procedure. Interestingly, alloyed cutting tools were created back in the 19th century by the scientist Muschette, along with the creation of metal-cutting machines. And Robert Hadfield put production on an industrial footing already in the 20th century, and now this composition is used everywhere. At the same time, the brand developed at that time suffered virtually no changes in the recipe. Only minor modifications are made that are prepared specifically for special applications, such as resistance to extreme low or high temperatures.
Alloy steel is an alloy that contains a large amount of impurities that increase strength, ductility, corrosion resistance and other properties. It is actively used for the manufacture of tools and semiconductors, since not only mechanical characteristics are taken into account, but also conductive ones.
When creating, it is important not only what substances are added as impurities (aluminum, nickel, chromium, etc.), but also the production technology. Depending on the predominant alloying additive, the grades have names - chromium steel, chromium-nickel, chromium-vanadium, etc. The use of steel structures and parts occurs in almost all production areas - from ordinary household construction to the oil and metallurgical industries.
The process and basics of alloying steels
There are two main methods:
- Superficial. In such a situation, only the top layer is doped with additives - its width depends on many factors, including the required characteristics. On average, the thickness does not exceed 1-2 mm. This forms a film on the surface that has the necessary properties, for example, anti-friction. This option is relatively inexpensive, but of high quality (better than, for example, spraying). It is used not only for metals, but also for working with ceramic and glass products.
- Volumetric. It involves the introduction of additional substances directly into the entire volume of the alloy. The process can be carried out at various stages of smelting with the addition of various elements - both metals and non-metals, the most common of which is phosphorus.
Changes occur at the microstructural level. They, in turn, change the physical and chemical characteristics of the entire steel element.
Separately, it is worth talking about doping semiconductors. It is carried out using the following methods:
- thermal diffusion - a temperature difference is used for the diffusion process;
- neutron transmutation process - actively used for silicon and semiconductors;
- ion implantation - ion beams are bombarded into the surface layer.
Thus, regardless of what is used (nuclear reactions, heat or ion energy), there are several stages of the process - preparatory, application of a layer of various additives, as well as finishing, which consists of additional exposure.
Alloy steels
Elements whose content exceeds the normal limit specified in the standards are called alloying additives. Changing the chemical composition of a metal by introducing alloying additives is called steel alloying. Main purposes of alloying:
- increased hardenability;
- obtaining specific strength properties;
- inducing desired structural changes;
- obtaining special chemical or physical properties;
- improvement and simplification of heat treatment technology;
- increasing corrosion resistance and resistance to various temperatures.
Based on the above, it follows that steel alloying is a metallurgical smelting process during which various additives are introduced into it. Alloying elements are added in two ways:
- Volumetric - components penetrate into the deep structure of the material by adding them to the charge or melt.
- Surface - introduction of alloying components only to the top layer of steel, to a depth of 1-2 mm. This method gives the material certain properties, for example, anti-friction.
Properties and purpose: for what purpose is steel alloying carried out?
With the development of industry, the number of necessary varieties of metal compositions is actively increasing. Depending on what properties need to be obtained, different elements can be added - chromium, silicon, copper, etc. How different the properties of these substances are, so varied are the effects obtained. It is very important to achieve the required proportions. It is by this property that all alloys are classified - according to the basic impurity, and those components that are found in the smallest quantities are called secondary ingredients.
The iron used as a basis is actually not very strong. It needs processing and improvement. The most standard, familiar method is the addition of carbon during heating, followed by rapid cooling. And depending on the percentage of this substance (from 0.1 to 1.15 percent of the composition), one can distinguish between soft, semi-soft, semi-hard and hard steel.
Risks of alloying
Unfortunately, any chemical additives under certain conditions can be not so much beneficial as have a negative effect. For example, one component that increases hardness can simultaneously increase brittleness. There are several other threats, here they are:
- most ferroalloys are manufactured in very small particles, in fact it is metal dust, which is explosive - fire, toxicity, explosions, all this can lead to increased risks;
- fumes that can be formed during production processes have a negative impact on health - the smallest dust particles can settle on the lungs;
- if tin is added to the alloy in combination with lead, then you need to be especially careful when heating, since the composition is toxic when exposed to high temperatures.
Practical application: what alloying steel does
There are so many characteristics obtained that it all depends on the specific case. We will give several specific situations:
- Increased hardness. This is necessary especially for basic metal structures so that they can withstand very high, especially static loads. Platinum is often added for this purpose.
- Ferromagnetic properties. To ensure that iron loses its magnetic properties, the alloy must contain cobalt.
- To prevent silver from tarnishing and corrosion, rhodium can be added. It may also be supplemented with palladium or platinum to increase its strength.
- The use of copper as an alloying additive increases corrosion resistance. The second application is for silver items, since silver itself is too soft.
- Increased hardness and strength without changing the level of ductility. Perhaps when the ions of the iron crystal lattice are replaced by atoms of the alloying element.
- Dissolution of certain non-metals in the composition leads to the fact that they literally displace harmful impurities that significantly affect the quality of products.
- Changing the grain size of the alloy. This may cause an increase in ductility and slight anisotropy after rolling.
This is an incomplete list of situations during which this procedure is used.
The purpose and application are very diverse. One of the main ones can be noted - the manufacture of tools for metalworking. Depending on the use, all methods of alloying steels are divided into three types - structural, instrumental and special purpose.
Ferrous alloys
These are metals that are based on iron. A common option is cast iron, which, due to its high carbon content, is not only very strong, but also brittle. This entire category does not have the highest mechanical properties (except for selected steel), but due to its low cost, as well as due to its fairly simple production by casting, all ferrous alloy materials have a very large production.
Non-ferrous alloys
These are compositions based on all other metals except iron. All of them are divided into light and heavy. The former have a low density of up to 5 mg per cubic centimeter. They are based on magnesium, titanium and aluminum. The latter, on the contrary, are denser (from 5 mg/cm3 and higher), they are based on copper and zinc. They include bronze - tin and tin-free - and brass. Almost all of the listed materials have the following characteristics:
- resistance to corrosion, which allows the alloy to be used even in conditions of high humidity and with constant contact with oxygen;
- high thermal conductivity and electrical conductivity - this is what allows the substance to be used in the manufacture of electrical parts, elements, contacts, wires;
- low density and, as a result, weight;
- simple and streamlined manufacturing process.
Stainless steel
The well-known stainless steel also belongs to alloy steels. It is so universal that it is used literally everywhere - from the manufacture of ordinary dishes for household use to specific branches of metallurgy. The main feature of the composition, which lies in its name, is corrosion resistance. But besides this, there are several more special characteristics:
- Aesthetic appearance. Since it is possible to use steel alloying with different essence of technological processes, it is possible to obtain a surface with qualitatively different characteristics. It can be a glossy sheen or a matte reflection, or an engraved finish. It is very easy to apply a pattern to the top layer, as well as color it. All this allows the material to be used not only for production purposes, but also for decorative finishing of premises and when creating furniture.
- Excellent mechanical properties. High strength, wear resistance, resistance to strong temperature changes, elasticity, impact resistance - all this makes the products applicable in a large production area. It is especially worth noting that at low temperatures (frost) the fragility does not increase, so you can work with stainless steel even in winter.
- Fire resistance. This quality is revealed due to the high melting point - up to 800 degrees. Therefore, even with constant contact with fire, no toxic fumes are released, and no deformation occurs.
- Corrosion resistance. As we noted, one of the main properties. This is achieved by the fact that the alloy contains chromium in a fairly large amount - from 10.5%. It reacts chemically with oxygen and leads to the formation of an oxide film. It is this oxide that protects against rust.
There are also some disadvantages. For example, it is quite difficult to process stainless steel. Many people note difficulties in forming a weld.
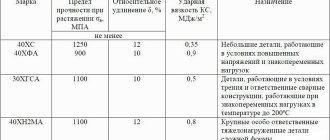
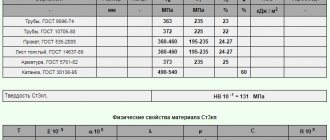
Alloying elements. Alloy steels, their markings.
Elements that are specially introduced into an alloy in order to change its structure and properties are called alloying, and this alloy is alloyed . To alloy steels, a significant number of elements of the periodic table are used (Table 2.4.).
Alloying elements have the most significant effect on the properties of steels, affecting the polymorphism of iron. The temperature of polymorphic transformations of iron depends on all the elements dissolved in it. In their presence, the range of existence of g-iron expands. When a certain amount of alloying elements is introduced, the g-state region ranges from room temperature to the melting point. Such alloys are called austenitic. Other elements (V, Si, Mo, etc.) make ferrite stable up to the melting point. Such alloys are called ferritic. When heated and cooled, the eutectoid transformation does not occur in them.
Alloying elements can be present in steels in a free state, in the form of chemical compounds with iron or with each other, in the form of oxides, sulfides and other non-metallic impurities, in the carbide phase, and also in the form of solid solutions in iron. Most often they dissolve in the main phases of iron-carbon alloys (ferrite, austenite, cementite) or form special carbides.
Carbide-forming elements (molybdenum, vanadium, tungsten, titanium) delay the release of carbides during tempering and increase the structural strength of steel.
The influence of alloying elements on the properties of steels is manifested, first of all, in changes in the properties of ferrite, dispersion of the carbide phase, hardenability, grain size, etc. By volume (more than 90%), ferrite is the main component of structural steels. Alloying elements dissolve in it, replacing iron atoms in the lattice and distorting it, which leads to an increase in the strength and hardness of ferrite. The increase in the latter is most strongly facilitated by the introduction of silicon, manganese and nickel. Most alloying elements, however, reduce the viscosity of ferrite and increase the threshold of its cold brittleness. The exception is nickel, which has the most favorable effect on the properties of steel. Chromium and nickel are the main alloying components of stainless steels (Table 2.4.).
Table 2.4.
The influence of alloying elements on the properties of steels.
| Alloying element | Designation | Properties imparted to steels | Examples of steel grades |
| Nitrogen (N) | A | Treatment in a nitrogen atmosphere (nitriding) leads to the formation of a solid solution in ferrite and nitride compounds, which imparts hardness to the surface layers | |
| Niobium (Nb) | B | Niobium - increases the acid resistance of steels | 03Х16Н15М3Б |
| Tungsten (W) | IN | Tungsten increases hardness and red-hardness, and the ability to maintain wear resistance at high temperatures. Tungsten gives steel its toughness. | В18 В6М5К5 |
| Manganese (Mn) | G | Manganese - with a content of more than 1 percent, it increases hardness, wear resistance, and resistance to shock loads. Manganese in the form of ferromanganese is used to “deoxidize” steel during its melting, i.e., to remove oxygen from it. Binds sulfur, which also improves the properties of steels. Sometimes in combination with other alloying metals, it greatly strengthens the steel, making it hard and resistant to wear and impact (the steel sharply strengthens and becomes harder upon impact). This steel is used for the manufacture of ball mills, earthmoving and stone crushing machines, armor elements, etc. | 14Г2 ШХ15ГС 30ХГС-Ш А40Г |
| Copper (Cu) | D | Copper - reduces corrosion of steels | 10Х18Н3Г3Д2Л |
| Cobalt (Co) | TO | Cobalt - increases heat resistance, magnetic permeability | R6M5K5 |
| Molybdenum (Mo) | M | Molybdenum - increases red resistance, strength, corrosion resistance at high temperatures. Molybdenum is used for alloying steels, as a component of heat-resistant and corrosion-resistant alloys. | R6M5K5 03Х16Н15М3Б |
| Nickel (Ni) | N | Nickel - increases strength, ductility, corrosion resistance. The introduction of a sufficient amount of nickel (Ni) into chromium steel provides better mechanical strength, makes the steel more resistant to corrosion (stainless steel) and low temperatures. | 03Х16Н15М3Б 12Х2Н4А |
| Phosphorus (P) | P | Increases fluidity, fragility | |
| Boron (B) | R | Increases the hardenability of steel, making steel sensitive to overheating. | |
| Silicon (Si) | WITH | Gives strength, increases toughness, promotes deoxidation. | 30ХГС-Ш 60С2ХFA 33ХС 38ХС |
| Titanium (Ti) | T | Increases strength, corrosion resistance | |
| Vanadium (V) | F | Increases density, strength, impact and abrasion resistance. Slows down the aging of steel. | 9Х2МФ |
| Chromium (Cr) | X | Increases hardness and corrosion resistance. Chromium steels, compared to carbon steels, have higher strength properties with some lower ductility in the core and better strength in the cemented layer; sensitive to overheating, hardenability is low. With the introduction of alloying elements, a sudden increase in corrosion resistance occurs. The steels weld well. | ШХ15ГС 30ХГС-Ш ШХ6 03Х16Н15М3Б 40Х |
| Zirconium (Zr) | C | Alloying steels with zirconium (up to 0.8%) increases their mechanical properties and machinability. | |
| Aluminum (Al) | YU | Aluminum - increases scale resistance Aluminizing imparts corrosion and scale resistance to steel and other alloys. Increases the heat resistance of alloys based on iron, copper, titanium and some other metals. Slows down the aging of steel. | AK7M2AK21M2 |
| Rare earth metals | H | Used to bind sulfur and phosphorus into refractory compounds |
The classification of alloy steels is based on four characteristics: chemical composition, equilibrium structure (after annealing), structure after cooling in air (after normalization), purpose.
Depending on the introduced elements, alloy steels are divided into chromium, manganese, chromium-nickel, chromium-nickel-molybdenum. A variation of the classification by chemical composition is the classification by quality. Alloy steels are divided into high-quality (up to 0.04% S and up to 0.035% P), high-quality (up to 0.025% S and up to 0.025% P) and especially high-quality (up to 0.015% S and up to 0.025% P) (section 2.5. classification of steels ).
According to the type of equilibrium structure.
Based on this feature, steels are divided into hypoeutectoid, eutectoid, hypereutectoid and ledeburite. Eutectoid steels have a pearlitic structure, while hypoeutectoid and hypereutectoid steels, along with pearlite, respectively, contain excess ferrite or secondary carbides of the M3C type. Thus, taking into account phase equilibrium, alloy steels are classified into pearlitic, carbide, ferritic or austenitic classes.
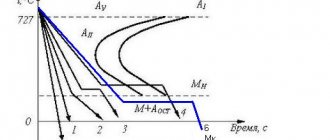
According to the structure after normalization. Here it is assumed that steels are divided into three main classes: pearlitic, martensitic and austenitic.
This division is due to the fact that with an increase in the content of alloying elements in the steel, the stability of austenite in the pearlitic region increases (this is manifested in a shift to the right of the C-shaped curves); At the same time, the temperature range of martensitic transformation decreases. All this leads to a change in the structures obtained during normalization from pearlite (sorbitol, troostite and bainite) in relatively low-alloy steels to martensite (in alloyed steels) and austenite (in high-alloy steels).
By appointment. According to their purpose, steels are divided into structural (for example, cemented, improved), instrumental and with special properties. The latter include “automatic”, spring, ball-bearing, wear-resistant, corrosion-resistant, heat-resistant, heat-resistant, electrical and other steels. “Special properties” of steel can be physical, for example, with certain magnetic characteristics or a low coefficient of linear expansion: electrical steel, superinvar, chemical, for example, stainless, heat-resistant, heat-resistant steels.
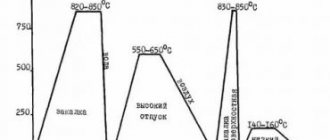
Heat-resistant steels and alloys. Heat-resistant, or scale-resistant, includes steels that ensure the operation of products at temperatures above 500 ° C for a given time (their detailed description is given below, in section 2.13).
Based on the content of alloying elements, heat-resistant steels and alloys are divided into low-, medium- and high-alloy.
Loaded parts of installations with a working medium temperature of 450 – 470 °C are made of chromium steels. To improve performance characteristics, vanadium, tungsten, molybdenum, niobium, and titanium are introduced into the steel composition. These elements, forming carbides and Laves phases, increase the heat resistance of steel. Alloying with boron, zirconium, cerium, as well as nitriding further increases its heat resistance (section 2.13).
Tool steels and hard alloys. Low-alloy steels with low hardenability are used for the manufacture of tools operating at temperatures up to 200 – 260 °C. Tools of large sizes and complex shapes can be made from such steels.
Low alloy steels are produced in the form of rods, strips and rods with improved surface finish quality. For the manufacture of high-performance tools designed to work at high cutting speeds, high-speed steels are used. The main advantage of high-speed steels is their high heat resistance, which is ensured by the introduction of a significant amount of carbide-forming elements: W, Mo, V, Co. High-speed steels retain their martensitic structure up to temperatures of 600 – 640 °C, which makes it possible to increase the cutting speed by 3-5 times compared to machining with conventional tools. The alloying elements contained in high-speed steels cause a decrease in the critical hardening rate.
The cost of high-speed steels is approximately 5-6 times higher than the cost of alloy tool steels. Therefore, tools made from them are used primarily for cutting high-strength and difficult-to-cut materials.
Marking of alloy steels. Alloying steels are marked with numbers and letters indicating the approximate composition of the steel. At the beginning of the brand there are two-digit numbers (for example, 12ХН3А), indicating the average carbon content in hundredths of a percent. Russian letters to the right of the number indicate alloying elements included in the steel (Table 2.5.).
If there is a number after the letter indicating the alloying element, then it indicates the content of this element as a percentage. If there is no number, then the steel contains 0.8 - 1.5% of the alloying element, with the exception of molybdenum and vanadium (the content of which in steels is usually up to 0.2 - 0.3%), as well as boron (in steel with the letter P it must be at least 0.0010%).
High-quality and extra-high-quality steels are marked in the same way as high-quality ones, but at the end of the high-quality steel grade they put the letter A (this letter in the middle of the brand designation indicates the presence of nitrogen specially introduced into the steel), and after the extra-high-quality grade the letter “Ш” is separated by a dash. .
Certain groups of steels are designated somewhat differently.
Ball bearing steels are marked with the letters “ШХ”, after which the chromium content is indicated in tenths of a percent:
ШХ6 - ball bearing steel containing 0.6% chromium;
ШХ15ГС - ball bearing steel containing 1.5% chromium and from 0.8 to 1.5% manganese and silicon.
High speed steels. Designations of high-speed steel grades begin with the letter P and a number indicating the average tungsten content in the steel. This is followed by letters and numbers that determine the mass fractions of other elements. Unlike alloy steels, the names of high-speed steels do not indicate the percentage of chromium, because it is about 4% in all steels and carbon (it is proportional to the vanadium content). The letter F, indicating the presence of vanadium, is indicated only if the vanadium content is more than 2.5%.
P 6 M 5
– high-speed steel of composition 0.82 – 0.9% C, 3.8-4.4% Cr, 4.8-5.3% Mo, 1.7-2.1% V, 5.5-6, 5% W, and steel
P 6 AM 5F3
– high-speed steel containing 0.95 - 1.05% C, 3.8-4.3% Cr, 4.8-5.3% Mo, 2.3-2.7% V, 0.05-0 ,1%N, 5.7-6.7%W.
P18
– high-speed steel containing an average of 18.0% tungsten.
The designations of corrosion-resistant (stainless), heat-resistant and heat-resistant steels according to GOST 5632-72 consist of numbers and are based on the same principles as the designations of structural alloy steels. The letter L is added to the designation of cast corrosion-resistant steels of this type.
08Х18Н10Т
has a composition of 0.08% C, 17.0-19.0% Cr, 9.0-11.0% Ni, 0.5 -0.7% Ti, cast steel
16Х18Н12С4ТУЛ
has a composition of 0.13-0.19% C, 17.0 – 19.0% Cr, 11.0-13.0% Ni, 3.8-4.5% Si, 0.4-0.7% Ti, 0.13-0.35% Al.
Experimental steels smelted at , are initially designated by the letters EI (research electric steel) or EP (trial electric steel) with a development (mastering) serial number, for example EI962 (11Х11Н2В2МФ), ЭПЗЗ (10Х11Н23ТЗМР). This simplified designation of steels, especially high-alloy steels, is subsequently widely used in industrial settings.
When marking alloys based on iron-nickel, the quantitative content of nickel is indicated (in percent), listing only the letter designations of the remaining alloying elements, for example KhN38VT, KhN45MVTYUBR.
Examples of marking of alloy steels are given in Table 2.5, given in [https://splav.kharkov.com/choose_type.php].
03Х16Н15М3Б - high-alloy quality steel, calm steel contains 0.03% carbon, 16.0% chromium, 15.0% nickel, up to 3.0% molybdenum, up to 1.0% niobium.
30ХГС-III is an especially high-quality medium-alloy steel containing 0.30% carbon and from 0.8 to 1.5% chromium, manganese and silicon each. 60S2HFA - high-quality alloy steel, calm, contains approximately 0.60% carbon, about 2% silicon, 0.8-1.5% chromium, up to 0.2% vanadium.
Table 2.5.
Types of steels, their grades, composition, applications (examples).
| steel grade | Compound | Application |
| Carbon tool steel U7, U7A, U8, U8A, U8G, U8GA, U9, U9A, U10, U10A, U11, U11A, U12, U12A, U13A | ||
| U10 | 0.96-1.03% C, 0.17-0.33% Si and Mn, up to 0.25% Ni, up to 0.028% S, up to 0.03% P, up to 0.2% Cr, up to 0 .25% Cu | tools working in conditions that do not cause heating of the cutting edge: hand taps, rasps, needle files, saws for woodworking, dies for cold stamping, smooth gauges, axes |
| U13A | 1.26-1.34% C, 0.17-0.33% Si and Mn, up to 0.25% Ni, up to 0.028% S, up to 0.03% P, up to 0.2% Cr, up to 0 .25% Cu | tools with increased wear resistance, operating at moderate and high pressures without heating the cutting edge |
| Heat-resistant alloy 10Х15Н35В3ТУ; XN35VTR; ХН45У; ХН56ВМКУ; ХН60У; KhN70VMTYUF; ХН32Т; ХН65ВМТУ; ХН75ВМУ; ХН78Т | ||
| XN35VTR | 37.725-45.9% Fe, up to 0.1% C, up to 0.6% Si, up to 1% Mn, 35-38% Ni, up to 0.02% S, up to 0.03% P, 14-16 % Cr, 4-5% W, 1.1-1.5% Ti, up to 0.025% Se | sheet for housings and guide blades of turbines operating at temperatures up to 750-800° |
| ХН56ВМКУ | Up to 1.5% Fe, up to 0.1% C, up to 0.6% Si, up to 0.3% Mn, 52.235-62.6% Ni, up to 0.01% S, up to 0.015% P, 8, 5-10.5% Cr, up to 0.02% Ce, 6.5-8.5% Mo, 6-7.5% W, 11-13% Co, 5.4-6.2% Al, up to 0.02% B | turbine blades |
| Casting steel with special properties 07Х17Н16ТЛ; 08Х15Н4ДМЛ; 10X12NDL; 10Х18Н3Г3Д2Л; 120G10FL; 12Х25Н5ТМФЛ; 15Х18Н22В6М2Л; 15Х13Л; 10Х18Н9Л; 07Х18Н9Л. | ||
| 10Х18Н3Г3Д2Л | Up to 0.1% C, up to 0.6% Si, 2.3-3% Mn, 3-3.5% Ni, up to 0.03% S and P, 17-19% Cr, 1.8-2 .2% Cu | for cavitation-resistant parts of the working part of hydraulic turbines operating at pressures not exceeding 80 l/h in sections up to 300 mm; austenitic-ferritic steel |
| 12Х25Н5ТМФЛ | Up to 0.12% C, 0.2-1% Si, 0.3-0.8% Mn, 5-6.5% Ni, up to 0.03% S and P, 23.5-26% Cr, 0.06-0.12% Mo, 0.07-0.15% V, 0.08-0.2% N and Ti, up to 0.3% Cu | for parts that are heat-resistant at temperatures up to 600 degrees C, as well as parts operating under pressure up to 30 MPa; austenitic-ferritic steel |
| Ordinary steel for castings 03Н12Х5М3ТЛ; 110G13L; 12Х7Г3СЛ; 15GL; 20GL; 20L; 20HML; 25L; 27Х5ГСМЛ; 12DN2F. | ||
| 110G13L | 0.9-1.4% C, 0.8-1% Si, 11.5-15% Mn, up to 1% Ni, up to 0.05% S, up to 0.12% P, up to 1% Cr, up to 0.3% Cu | housings of vortex and ball mills, cheeks and cones of crushers, teeth and front walls of excavator buckets, railway crosses and other heavily loaded parts operating under static and high dynamic loads and from which high wear resistance is required. |
| 20L | 0.17-0.25% C, 0.2-0.52% Si, 0.35-0.9% Mn, up to 0.3% Ni, up to 0.045% S, up to 0.04% P, up to 0.3% Cr and Cu | Chabots, fittings, shaped castings of general mechanical engineering parts produced by the lost-wax method, parts of welded-cast structures and other parts operating at temperatures from -40 to 450 °C. |
| High-alloy heat-resistant steel 08Х15Н24В4ТР; 08Х20Н14С2; 09X16N16MV2BR; 10Х11Н20Т3Р; 10Х15Н25М3В3ТУК; 10Х7МВФБР; 12X25N16G7AR; 13Х12Н2В2МФ; 15Х18СУ; 18Х12ВМБФР. | ||
| 08Х15Н24В4ТР | 50.4-58.1% Fe, up to 0.08% C, up to 0.6% Si, 0.5-1% Mn, 22-25% Ni, up to 0.02% S, up to 0.035% P, 14-16% Cr, up to 0.025% Ce, 4-5% W, 1.4-1.8% Ti, up to 0.005% B | working and guide blades, fasteners, gas turbine disks with a long service life at temperatures of 650-700 degrees C; austenitic steel |
| 18Х12ВМБФР | 0.15-0.22% C, up to 0.5% Si and Mn, up to 0.06% Ni, up to 0.025% S, up to 0.03% P, 11-13% Cr, 0.4-0, 6% Mo, 0.15-0.18% V, 0.4-0.7% W, 0.2-0.4% Nb, up to 0.003% B | steam turbine blades, pipes and fasteners for long service life at temperatures up to 620 deg.C; martensitic-ferritic steel |
| Low-alloy heat-resistant steel 12MX; 15Х5ВФ; 15ХМФКР; 12Х1МФ; 15Х1М1Ф; 15Х5М; 16GNM; 12Х2МФБ; 15ХМ. | ||
| 15ХМФКР | 0.12-0.18% C, 0.17-0.37% Si, 0.4-0.7% Mn, up to 0.03% P and S, 1-1.3% Cr, 0.9 -1.2% Mo, 0.2-0.32% V, 1.3-1.5% Co, 0.006-0.01% B | forgings of turbine parts operating at a temperature of 580-600 degrees; steam pipes, collector pipes, steam superheating pipes |
| 15ХМ | 0.11-0.18% C, 0.17-0.37% Si, 0.4-0.7% Mn, up to 0.3% Ni, up to 0.035% S, up to 0.035% P, 0.8 -1.1% Cr, 0.4-0.55% Mo, up to 0.3% Cu | sections, forgings, pipes for superheaters, steam pipelines, manifolds, flanges, long-term operating at temperatures up to 500 degrees. |
| Heat-resistant, relaxation-resistant steel 20Х1М1Ф1БТ; 25Х1М1Ф; 30ХМА; 20Х1М1Ф1ТР; 25Х1МФ; 35ХМ; 20Х3МВФ; 25Х2М1Ф; 38Х2МУА; 20KhMFBR. | ||
| 25Х1М1Ф | 0.22-0.29% C, 0.17-0.37% Si, 0.4-0.7% Mn, up to 0.26% Ni, up to 0.025% S, up to 0.03% P, 1 .5-1.8% Cr, 0.6-0.8% Mo, 0.15-0.3% V, up to 0.2% Cu | solid forged rotors, shafts, disks and other parts operating at temperatures up to 540 degrees, fasteners for operating at temperatures up to 525 degrees. |
| 35ХМ | 0.32-0.4% C, 0.17-0.37% Si, 0.4-0.7% Mn, up to 0.3% Ni, up to 0.035% S and P, 0.8-1, 1% Cr, 0.15-0.25% Mo, up to 0.3% Cu | shafts, gears, spindles, pins, flanges, disks, tires, rods and other critical parts operating under heavy loads and speeds at temperatures up to 450-500 °C |
| Alloy tool steel 05Х12Н6Д2МФСГТ; 13X; 5ХВ2СФ; 6Х4М2ФС; 8Х6НФТ; 9Х5ВФ; 9HFM; ХВ4Ф; 9G2F; 6Х3МФС. | ||
| 5ХВ2СФ | 0.45-0.55% C, 0.8-1.1% Si, 0.15-0.45% Mn, up to 0.35% Ni, up to 0.03% S and P, 0.9- 1.2% Cr, 1.8-2.3% W, 0.15-0.3% V | knives for cold cutting of metal, thread-rolling dies, punches and crimping dies for cold work; woodworking tools for long-term work |
| ХВ4Ф | 1.25-1.45% C, 0.15-0.35% Si, 0.15-0.4% Mn, up to 0.35% Ni, up to 0.03% S and P, 0.4- 0.7% Cr, up to 0.5% Mo, 3.5-4.3% W, 0.15-0.3% V, up to 0.3% Cu | cutters and milling cutters for low-speed cutting of hard metals (rolls with a hardened surface), engraving cutters for very hard work, piercing punches, etc. |
| Die tool steel 27Х2Н2М1Ф; 3Х3М3Ф; 4Х3ВМФ; 4Х5МФС; 5Х3В3МФС; 6ХВ2С; 7ХГ2ВМ; X12; 5X2MNF; 4Х2НМФ. | ||
| X12 | 2-2.2% C, 0.1-0.4% Si, 0.15-0.45% Mn, up to 0.35% Ni, up to 0.03% S and P, 11.5-13% Cr, up to 0.2% Mo and W, up to 0.15% V, up to 0.03% Ti, up to 0.3% Cu | cold dies of high resistance to abrasion, not subject to strong impacts and shocks; drawing boards, eyes for calibrating bar metal for thread rolling, bending and forming dies, complex sections of body dies, dies and punches of cutting and perforating dies, stamping of the active part of electrical machines, etc. |
| 5Х2МNF | 0.46-0.53% C, 0.1-0.4% Si, 0.4-0.7% Mn, 1.2-1.6% Ni, up to 0.03% S and P, 1 .5-2% Cr, 0.8-1.1% Mo, 0.3-0.5% V | for large-sized solid dies (diameter up to 600 mm) for stamping forgings from structural steels and heat-resistant alloys on hammers and crank presses; clamping and forming inserts, typesetting and forming punches for heading structural steels and heat-resistant alloys on horizontal forging machines; hot cutting knives |
| High-speed tool steel 11M5F; P12; R18K5F2; R2M5; R6M5F3; R9M4K8; 11Р3АМ3Ф2; R12F3; R18F2; P9. | ||
| R18K5F2 | 0.85-0.95% C, up to 0.5% Si and Mn, up to 0.4% Ni, up to 0.03% S and P, 3.8-4.4% Cr, up to 1% Mo, 17-18.5% W, 1.8-2.2% V, 4.7-5.2% Co | for roughing and semi-roughing tools when processing high-strength, stainless and heat-resistant steels and alloys |
| R9M4K8 | 1-1.1% C, up to 0.5% Si and Mn, up to 0.4% Ni, up to 0.03% S and P, 3-3.6% Cr, 3.8-4.3% Mo , 8.5-9.5% W, 2.3-2.7% V, 7.5-8.5% Co | for processing high-strength stainless and heat-resistant steels and alloys under conditions of increased heating of the cutting edge: gear cutting tools, cutters, shaped cutters, countersinks, taps |
| Rolling tool steel 45ХНМ; 60ХН; 75HSMF; 9X2; 55X; 60ХСМФ; 7Х2СМФ; 9Х2МФ; 60Х2СМФ; 75ХМ. | ||
| 9Х2МФ | 0.85-0.95% C, 0.25-0.5% Si, 0.2-0.7% Mn, up to 0.5% Ni, up to 0.03% S and P, 1.7- 2.1% Cr, 0.2-0.3% Mo, 0.1-0.2% V | work rolls for cold metal rolling mills under particularly difficult operating conditions, work rolls for wire crimping and section mills |
| 75ХМ | 0.7-0.8% C, 0.2-0.6% Si, 0.2-0.7% Mn, up to 0.5% Ni, up to 0.03% S and P, 1.4- 1.7% Cr, 0.2-0.3% Mo | work and support rolls of sheet mills for hot rolling of ferrous metals, support rolls of two and four-roll stands of sheet mills for cold rolling of metals |
| Low-alloy structural steel for welded structures 06G2SYU; 09G2D; 10G2BD; 10GT; 12G2B; 12HGN2MFBAYU; 14HGS; 15G2SFD; 08G2S; 10G2S1. | ||
| 14ХГС | 0.11-0.16% C, 0.4-0.7% Si, up to 0.3% Ni, up to 0.04% S, up to 0.035% P, 0.5-0.8% Cr, up to 0.3% Cu, up to 0.08% As | electric welded pipes of high pressure gas pipelines; welded structures, sheet metal, valve structural parts |
| 10G2S1 | Up to 0.12% C, 0.9-1.2% Si, 1.3-1.65% Mn, up to 0.3% Ni, up to 0.04% S, up to 0.035% P, up to 0.3 % Cr and Cu, up to 0.008% N, up to 0.08% As | boiler drums, pressure vessels and other boiler parts operating at temperatures up to 450 degrees. |
| Structural bearing steel 11Х18М-ШД; ShH20SG; 8Х4В9Ф2-Ш; ШХ4; ШХ15; SHH15SG. | ||
| 8Х4В9Ф2-Ш | 0.7-0.8% C, up to 0.4% Si and Mn, up to 0.03% S and P, 4-4.6% Cr, 8.5-9.5% W, 1.4- 1.7%V | for the production of rolling bearings operating in aggressive environments |
| SHH15SG | 0.95-1.05% C, 0.4-0.65% Si, 0.9-1.2% Mn, up to 0.3% Ni, up to 0.02% S, up to 0.027% P, 1 .3-1.65% Cr, up to 0.25% Cu | large-sized rings of ball and roller bearings with walls more than 20-30 mm thick, balls with a diameter of more than 50 mm; rollers with a diameter of more than 35mm |
| Alloy structural steel 10G2; 12ХН; 14Х2ГМР; 15N2M; 15HF; 18Х2Н4МА; 20G; 20Х2Н4А; 15ХА; 12ХН3А. | ||
| 18Х2Н4МА | 0.14-0.2% C, 0.17-0.37% Si, 0.25-0.55% Mn, 4-4.4% Ni and Cu, up to 0.025% S and P, 1.35 -1.65% Cr, 0.3-0.4% Mo, up to 0.3% Cu | in a cemented and improved state, it is used for critical parts that require high strength, toughness and wear resistance, as well as for parts subjected to high vibration and dynamic loads. Steel can be used at temperatures from -70 to +450 °C |
| 20Х2Н4А | 0.16-0.22% C, 0.17-0.37% Si, 0.3-0.6% Mn, 3.25-3.65% Ni, up to 0.025% S and P, 1.25 -1.65% Cr, up to 0.3% Cu | gears, pinion shafts, pins and other case-hardened, especially critical, highly loaded parts, which are subject to the requirements of high strength, ductility and toughness of the core and high surface hardness, operating under shock loads or at subzero temperatures |
| Structural steel with increased machinability A11; A35; A45E; AS14HGN; AS35G2; AS45G2; A12; A35E; AS30ХМ; A40XE; | ||
| A12 | 0.08-0.16% C, 0.15-0.35% Si, 0.7-1.1% Mn, 0.08-0.2% S, 0.08-0.15% P | axles, rollers, bushings, gears, pinions, pins, screws, bolts and other lightly loaded small parts of complex shape, processed on automatic machines, and which are subject to increased requirements for surface quality and dimensional accuracy |
| Precision alloy with high electrical resistance | ||
| Х27У5Т | Up to 0.05% C, up to 0.6% Si, up to 0.3% Mn, up to 0.6% Ni, up to 0.015% S, up to 0.02% P, 26-28% Cr, up to 0.1 % Ce and Ca, 0.15-0.4% Ti, 5-5.8% Al, up to 0.5% Ba | for electric heating elements of furnaces with a maximum operating temperature of 1350 °C |
(https://splav.kharkov.com/choose_type.php).
Classification of alloy steels
There are three degrees of doping, according to which the percentage of additive substances changes. From here the material can be:
- low-alloy – up to 2.5% impurities in the composition;
- medium alloyed – up to 10%;
- highly alloyed - up to 50% additives.
The molecular structure also differs; according to it, all alloys are classified into:
- martensitic - with completely the same grain size;
- ferritic;
- austenitic, as well as various types of combined steels.
Carbon is the most commonly used impurity; it is responsible for increased strength and impact resistance. In this regard, alloys are classified:
- low carbon – up to 0.25% content;
- medium carbon – up to 0.65%;
- high carbon – more than 0.65%.
The structure also implies division into the following classes:
- hypoeutectoid - the alloy contains areas of ferrite;
- eutectoid - based on pearlite;
- ledeburite or hypereutectoid - with primary/secondary carbides.
We have also already noted that, according to purpose, everything is divided into:
- structural - they, in turn, are divided into construction and mechanical engineering;
- instrumental – for creating metalworking tools;
- with special properties, including resistant to temperature changes, fire-resistant and others.
Separately distinguish:
- heat-resistant - chromium, vanadium, molybdenum are added to them, they are used in the energy sector, as well as for other industries with high temperatures;
- improved - they are additionally subjected to heat treatment, usually hardening, they are characterized by increased strength and sensitivity to stress concentration;
- case-hardened - they are first carburized and then hardened; they are excellent for the production of gears, shafts and other elements for which wear resistance is important;
- high-speed cutting – very high hardness and red resistance up to a high temperature limit;
- stainless – have an oxide film coating that prevents rusting;
- with improved magnetic or electrical properties.
If we classify alloy steels for construction purposes in more detail, we distinguish:
- mass – used virtually everywhere;
- bridge construction;
- shipbuilding - very resistant to brittle fracture;
- for hot water supply and steam – classified as heat-resistant;
- low-low – actively used in aircraft construction, etc.
In addition, all alloys can be classified according to the main impurity, and also divided into two-component, three-component, and so on according to a specific recipe.
Application
Due to such characteristics as strength, load resistance, hardness, reduced magnetization and the desired level of toughness, alloy steel is used in a wide variety of areas of human activity. From it they produce:
- medical instruments, including cutting ones;
- parts with high supporting and radial loads;
- elements of metalworking machines;
- stainless steel utensils;
- car parts;
- aerospace parts;
- molds and other elements for hot stamping that retain their properties at temperatures up to + 600 degrees;
- measuring instruments and so on.
Marking of alloy steels
Since this class of materials is very broad, there was a need to designate individual elements. Unfortunately, there are no uniform rules throughout the world on how to place a mark. We will list the rules specific to Russian production.
The markings are based on numbers and letters. Letters can mean special properties or belonging to a narrow class, but most often they are responsible for a component that is in the composition:
- A – nitrogen.
- K – cobalt.
- C – silicon.
- T – titanium.
- E – selenium.
- B – niobium.
- G – manganese.
- M – molybdenum.
- P – phosphorus.
- F – vanadium.
- C – zirconium.
- B – tungsten.
- D – copper.
- N – nickel.
- X – chrome.
- R – boron.
- Yu – aluminum.
Russian state standard
GOST 4543-71 is responsible for marking. According to the document, by the letter that appears in front, you can determine which class the substance belongs to:
- F – stainless alloy.
- X – chromium.
- E – magnetic.
- I am chromium-nickel stainless steel.
- Ш – ball bearing.
- P – high-speed tool.
- A - high quality.
- N – obtained by hard-worked rolled steel.
- TO – method of heat treatment.
You should also look at the numbers. The first allows you to understand how much carbon is in the composition, and then, together with the letter, there is a percentage of the content of another alloying additive.
Here is an example of chromium compound labeling:
Stamps
In the CIS, alphanumeric marking of alloy steels is used. The letters indicate the main alloying additives, the numbers following the letters indicate the percentage of their content in the alloy (rounded to the nearest whole number). If the metal contains no more than 1.5% of one or another additive, the number is not given. The percentage of carbon × 100 is indicated at the beginning of the name of the steel. The letter A in the middle of the marking indicates the nitrogen content. If two A's appear at the end, this indicates extra pure steel. The letter Ш at the end denotes particularly high quality steel.
The marking may be supplemented with other designations, for example:
- E - electrical;
- P - high-speed;
- A - automatic;
- L - obtained by casting.
Comprehensive lists of alloy steel grades are specified in GOST 4543-71.
Difference Between Doping and Impurities
Additives are specifically introduced in significant quantities (more than 0.1% of the total composition), their influence on the result is high. While accidental impurities are inevitable, often harmful substances that are mixed in small quantities. And the higher the quality of the material, the fewer there are.
For example, chromium (up to 12%) is used to alloy steels for subsequent cold stamping - this is, of course, an additive. To complete the article, let's watch a few videos:
Alloying elements and their influence on the properties of steels
The marking of alloy steels indicates what additives it contains, as well as their quantitative value. But it is also important to know exactly what effect each of these elements has on the properties of the metal separately.
The addition of chromium increases corrosion resistance, increases strength and hardness, and is the main component in the creation of stainless steel.
The addition of nickel increases the ductility, toughness and corrosion resistance of steel.
Titanium reduces the graininess of the internal structure, increasing strength and density, improving machinability and corrosion resistance.
The presence of vanadium reduces the graininess of the internal structure, which increases fluidity and tensile strength.
The addition of molybdenum makes it possible to improve hardenability, increase corrosion resistance and reduce brittleness.
Tungsten increases hardness, prevents grains from expanding when heated, and reduces brittleness when tempered.
At contents of up to 1-15%, silicon increases strength while maintaining toughness. As the percentage of silicon increases, magnetic permeability and electrical resistance increase. This element also increases elasticity, corrosion resistance and oxidation resistance, but also increases fragility.
The introduction of cobalt increases impact resistance and heat resistance.
The addition of aluminum improves scale resistance.
Table of purpose of some types of steel
Separately, it is worth mentioning impurities and their effect on the properties of steels. Any steel always contains technological impurities, since it is extremely difficult to completely remove them from the steel composition. These types of impurities include carbon, sulfur, manganese, silicon, phosphorus, nitrogen and oxygen.
It has a very significant effect on the properties of steel. If it is contained up to 1.2%, then carbon helps to increase the hardness, strength, and yield strength of the metal. Exceeding the specified value contributes to the fact that not only strength, but also ductility begins to deteriorate significantly.
Features of alloying
Modern capabilities make it possible to smelt alloyed metals of any composition. Basic principles of the technology under consideration:
- Components are considered alloying only if they are introduced purposefully and the content of each exceeds 1%.
- Sulfur, hydrogen, phosphorus are considered impurities. Boron, nitrogen, silicon, and rarely phosphorus are used as nonmetallic inclusions.
- Bulk alloying is the introduction of components into a molten substance as part of metallurgical production. Surface is a method of diffusion saturation of the surface layer with the necessary chemical elements under the influence of high temperatures.
- During the process, additives change the crystal structure of the “daughter” material. They can create penetration or exclusion solutions, and can be placed at the boundaries of metallic and non-metallic structures, creating a mechanical mixture of grains. The degree of solubility of elements in each other plays a big role here.
Alloy copper
It is used in its pure form and as part of copper alloys, which have a wide variety depending on the ratio of the main and alloying elements: brass, bronze, cupronickel, silver and others.
Pure brass, an alloy with zinc, is not alloyed. If it contains alloying agents in a certain amount, it is considered multicomponent. Bronzes are alloys with other metal components; they can be tin or not containing tin; they are alloyed in all cases. Their quality is improved using Mn, Fe, Zn, Ni, Sn, Pb, Be, Al, P, Si.
The silicon content in copper compounds increases their corrosion resistance, strength and elasticity; tin and lead - determine anti-friction qualities and positive characteristics regarding machinability; nickel and manganese are components of so-called wrought alloys, which also have a positive effect on corrosion resistance; iron improves mechanical properties, and zinc improves technological properties.
They are used in electrical engineering as the main raw material for the manufacture of various wires, material for the manufacture of critical parts for chemical equipment, in mechanical engineering and instrument making, in pipelines and heat exchangers.
Historical path
The foundation for the development of alloying was laid by the justification of the crucible method of melting steel in Europe in the 18th century. In a more primitive version, crucibles were used in ancient times, including for the smelting of damask and Damascus steel. At the beginning of the 18th century, this technology was improved on an industrial scale and made it possible to adjust the composition and quality of the starting material.
- The simultaneous discovery of more and more new chemical elements pushed researchers into experimental smelting experiments.
- The negative influence of copper on the quality of steel has been established.
- Opened brass containing 6% iron.
Experiments were carried out from the point of view of the qualitative and quantitative influence of tungsten, manganese, titanium, molybdenum, cobalt, chromium, platinum, nickel, aluminum and others on the steel alloy.
The first industrial production of steel alloyed with manganese was established at the beginning of the 19th century. It has also been developed since 1856 as part of the Bessemer smelting process.
Alloying process
The main way to alloy steel is the method of volumetric metallurgical alloying. It consists of fusing the main element with alloying elements in various types of furnaces (induction, vacuum-arc, crucible, converters, arc, plasma, etc.). With this method, a significant loss of active substances (manganese, chromium, molybdenum, etc.) is possible.
There are also:
- mechanical alloying,
- recovery,
- electrolysis,
- plasmachemical reaction.
Mechanical alloying is performed in attritors - drums, in the center of which there is a shaft with cams. Powdered components are placed in them to obtain the desired alloy. During rotation, the cams “hit” the mixture, and the alloying additives are “driven” into the base.
In co-reduction, the oxides of the alloy elements are mixed with a reducing agent, for example, calcium hydride (CaH2) and heated. The reaction of reduction of oxides to metals occurs, and the diffusion process occurs simultaneously, leveling the composition of the alloy. The resulting calcium oxide (CaO) is washed with water, and the alloy (in powder form) goes into the next processing. Metallothermic reduction involves the use of metals (magnesium, calcium, aluminum, etc.) as reducing agents.
With the help of surface alloying, the surface of the product is given special properties. A certain element or alloy is applied to the top layer in the form of a small layer, then it is exposed to energy (laser radiation, plasma, high-frequency current, etc.) - the surface is melted, and a new alloy is formed on it.
Main purposes of alloying
The word "alloying" comes from the German "legieren" (to bind, to connect). The positive effect of alloying components on the properties of steel is associated with ensuring the occurrence of two physical and chemical processes.
Process No. 1
The formation of thermodynamic stable substitution solutions, accompanied by the replacement of part of the iron atoms (ions) in its crystal lattice (ions) of the alloying element. This leads to a distortion of the iron crystal lattice, since the radii of the ions (cations) of alloying elements differ from the radius of the iron cations, which increases the hardness and strength of iron while maintaining its ductility.
Process No. 2
The appearance of strong and practically insoluble chemical compounds in liquid iron between alloying additives introduced into the molten metal and non-metals dissolved in it (oxygen, nitrogen, sulfur, carbon, etc.).
The results of the formation of such compounds are:
- reduction of the residual content of dissolved non-metals in the molten metal, deteriorating its quality,
- reducing the total volume of harmful impurities (dissolved and in the form of non-metallic inclusions) in steel.
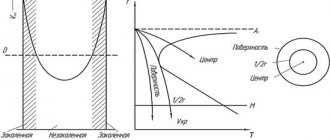
And also there is a release (precipitation) from the liquid metal of such small non-metallic inclusions, which serve as crystallization centers and lead to the formation of a fine-grained primary and secondary structure of steel. Due to this, it has better ductility, low anisotropy of properties after rolling, etc. Small non-metallic inclusions released during crystallization tend to accumulate on the surface of growing crystals, reducing the growth rate of the faces, and this, in turn, reduces the grain size of the steel.
Alloying in metallurgy
Story
Alloying began to be used purposefully relatively recently. This was partly due to technological difficulties. Alloying additives simply burned out when using traditional steel production technology. Therefore, to obtain Damascus (damascus) steel, they used a technology that was quite complex for those times.
It is noteworthy that the first steels with which man became acquainted were natural alloy steels. Even before the beginning of the Iron Age, meteorite iron containing up to 8.5% nickel was used.
Naturally alloyed steels made from ores initially rich in alloying elements were also highly valued. The increased hardness and toughness of Japanese swords with the ability to provide a sharp edge may be explained by the presence of molybdenum in the steel.
Modern views on the influence of various chemical elements on the properties of steel began to take shape with the development of chemistry in the second quarter of the 19th century.
Apparently, the first successful use of targeted alloying can be considered the invention in 1858 by Muschette of steel containing 1.85% carbon, 9% tungsten and 2.5% manganese. The steel was intended for the manufacture of cutters for metalworking machines and was the prototype of the modern line of high-speed steels. Industrial production of these steels began in 1871.
It is generally accepted that the first mass-produced alloy steel was Hadfield Steel, discovered by the English metallurgist Robert Abbott Hadfield in 1882. Steel contains 1.0 - 1.5% carbon and 12 - 14% manganese, has good casting properties and wear resistance. Without any significant changes in the chemical composition, this steel has survived to the present day.
Influence of alloying elements
To improve the physical, chemical, strength and technological properties, metals are alloyed by introducing various alloying elements into their composition. Chromium, manganese, nickel, tungsten, vanadium, niobium, titanium and other elements are used to alloy steels. Small additions of cadmium to copper increase the wear resistance of wires; additions of zinc to copper and bronze increase strength, ductility, and corrosion resistance. Alloying titanium with molybdenum more than doubles the operating temperature limit of the titanium alloy due to a change in the crystalline structure of the metal. Alloy metals may contain one or more alloying elements that give them special properties.
Alloying elements are introduced into steel to increase its structural strength. The main structural component in structural steel is ferrite, occupying at least 90% by volume in the structure. By dissolving in ferrite, alloying elements strengthen it. The hardness of ferrite (in the state after normalization) is most strongly increased by silicon, manganese and nickel. Molybdenum, tungsten and chromium have a weaker effect. Most alloying elements, while strengthening ferrite and having little effect on ductility, reduce its impact strength (with the exception of nickel). The main purpose of alloying is: increasing the strength of steel without the use of heat treatment by strengthening ferrite by dissolving alloying elements in it; increased hardness, strength and toughness as a result of increased stability of austenite and thereby increased hardenability; giving steel special properties, of which heat resistance and corrosion resistance are of particular importance for steels used in the manufacture of boilers, turbines and auxiliary equipment. Alloying elements can dissolve in ferrite or austenite, form carbides, produce intermetallic compounds, and be located in the form of inclusions without interacting with ferrite and austenite, as well as with carbon. Depending on how the alloying element interacts with iron or carbon, it affects the properties of steel differently. All elements dissolve to a greater or lesser extent in ferrite. The dissolution of alloying elements in ferrite leads to strengthening of steel without heat treatment. In this case, hardness and tensile strength increase, and impact strength usually decreases. All elements dissolving in iron change the stability of ferrite and austenite. The critical points of alloy steels shift depending on which alloying elements and in what quantities are present in it. Therefore, when choosing temperatures for quenching, normalizing and annealing or tempering, it is necessary to take into account the shift of critical points.
Manganese and silicon are introduced during the steelmaking process for deoxidation; they are technological impurities. Manganese is added to steel up to 2%. It is distributed between ferrite and cementite. Manganese significantly increases the yield strength, cold brittleness threshold, and hardenability of steel, but makes steel sensitive to overheating. In this regard, to grind grain with manganese, carbide-forming elements are introduced into the steel. Since the manganese content in all steels is approximately the same, its effect on steel of different composition remains imperceptible. Manganese increases strength without reducing the ductility of steel.
They are important in modern industry. Let's analyze some of them, highlight their distinctive and similar characteristics.
Additional classification
Alloyed structural alloys are suitable for the manufacture of machine parts and mechanisms in the engineering industry - they produce large-sized parts that are hardened and subjected to high tempering. Most alloying additives in steel increase hardenability. The introduction of additives should be sufficient, but not excessive. A high degree of doping can cause:
- decrease in plastic properties,
- development of temper brittleness,
- lowering the threshold of cold brittleness.
The exception is nickel; it shifts the threshold of cold brittleness to the region of low temperatures, so for machines operating in the North, mechanisms are made from nickel-containing steels. Spring alloy steel contains 0.5–0.7% carbon, and chromium, molybdenum and tungsten are added as additives. Such a composition should provide high resistance to small plastic deformations and high fatigue resistance.
Ball bearings - classified as hypereutectoid - carbon is about 1% with additional alloying of the metal with chromium (1.3–1.65%). In heat-resistant bearings, chromium is increased to 5%. Bearings are subject to special requirements for metallurgical cleanliness. The use of refining remelts, vacuum remelting methods, and treatment with synthetic slags make it possible to reduce the proportion and size of non-metallic inclusions, thereby increasing resistance to contact fatigue.