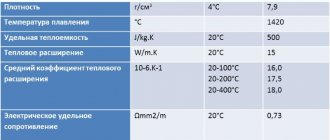
What kind of material is this?
Ordinary steel is a combination of iron and carbon and a number of impurities.
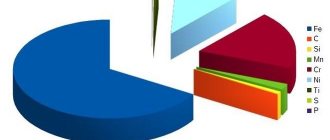
The definition of “alloy steel” (AS) means a special alloy, which is obtained by introducing a certain amount of chemical elements. This is done in order to obtain the necessary physical and chemical properties of the metal. As a rule, the following elements of the periodic table are added, which are difficult to do without depending on the specific situation:
- Nickel – H (Ni).
- Copper – M (Cu).
- Niobium – B (Nb).
- Chromium – X (Cr).
- Manganese – G (Mn).
- Silicon – C (Si).
- Vanadium – F (V).
- Tungsten – B (W).
- Molybdenum – M (Mo).
- Titanium – T (Ti).
- Aluminum – A (Al).
- Zirconium – C (Zr).
- Cobalt – K (Co).
But besides them, molybdenum and aluminum are used. Moreover, each of these elements is added for a specific purpose. And their quantity directly affects the obtaining of the necessary qualities. Now it becomes a little clear what alloy steel is.
Alloying elements and their influence on the properties of steels
The marking of alloy steels indicates what additives it contains, as well as their quantitative value. But it is also important to know exactly what effect each of these elements has on the properties of the metal separately.
Chromium
The addition of chromium increases corrosion resistance, increases strength and hardness, and is the main component in the creation of stainless steel.
Nickel
The addition of nickel increases the ductility, toughness and corrosion resistance of steel.
Titanium
Titanium reduces the graininess of the internal structure, increasing strength and density, improving machinability and corrosion resistance.
Vanadium
The presence of vanadium reduces the graininess of the internal structure, which increases fluidity and tensile strength.
Molybdenum
The addition of molybdenum makes it possible to improve hardenability, increase corrosion resistance and reduce brittleness.
Tungsten
Tungsten increases hardness, prevents grains from expanding when heated, and reduces brittleness when tempered.
Silicon
At contents of up to 1-15%, silicon increases strength while maintaining toughness. As the percentage of silicon increases, magnetic permeability and electrical resistance increase. This element also increases elasticity, corrosion resistance and oxidation resistance, but also increases fragility.
Cobalt
The introduction of cobalt increases impact resistance and heat resistance.
Aluminum
The addition of aluminum improves scale resistance.
Table of purpose of some types of steel
Separately, it is worth mentioning impurities and their effect on the properties of steels. Any steel always contains technological impurities, since it is extremely difficult to completely remove them from the steel composition. These types of impurities include carbon, sulfur, manganese, silicon, phosphorus, nitrogen and oxygen.
Carbon
has a very significant effect on the properties of steel. If it is contained up to 1.2%, then carbon helps to increase the hardness, strength, and yield strength of the metal. Exceeding the specified value contributes to the fact that not only strength, but also ductility begins to deteriorate significantly.
Manganese
If the amount of manganese does not exceed 0.8%, then it is considered a technological impurity. It is designed to increase the degree of deoxidation and also counter the negative effects of sulfur on steel.
Sulfur
When the sulfur content exceeds 0.65%, the mechanical properties of steel are significantly reduced, we are talking about a decrease in the level of ductility, corrosion resistance, and impact strength. Also, high sulfur content negatively affects the weldability of steel.
Phosphorus
Even a slight excess of phosphorus content above the required level is fraught with an increase in brittleness and fluidity, as well as a decrease in the toughness and ductility of steel.
Nitrogen and oxygen
When certain quantitative values in the steel composition are exceeded, inclusions of these gases increase brittleness and also contribute to a decrease in its endurance and toughness.
Hydrogen
Too much hydrogen content in steel leads to increased brittleness.
Historical path
The foundation for the development of alloying was laid by the justification of the crucible method of melting steel in Europe in the 18th century. In a more primitive version, crucibles were used in ancient times, including for the smelting of damask and Damascus steel. At the beginning of the 18th century, this technology was improved on an industrial scale and made it possible to adjust the composition and quality of the starting material.
- The simultaneous discovery of more and more new chemical elements pushed researchers into experimental smelting experiments.
- The negative influence of copper on the quality of steel has been established.
- Opened brass containing 6% iron.
Experiments were carried out from the point of view of the qualitative and quantitative influence of tungsten, manganese, titanium, molybdenum, cobalt, chromium, platinum, nickel, aluminum and others on the steel alloy.
The first industrial production of steel alloyed with manganese was established at the beginning of the 19th century. It has also been developed since 1856 as part of the Bessemer smelting process.
Doping and impurities - is there a difference?
From a formal point of view, some chemical elements contained in ordinary steels, both structural and ordinary quality, can also be called alloying. These include, for example, copper (up to 0.2%), silicon (up to 0.37%), etc.
Phosphorus and sulfur are constant companions of any steel. However, metal scientists mostly classify them not as alloying additives, but as impurities, although sometimes the percentage of another alloying element may be even smaller.
The reason is that any impurity is a consequence of either the purity of the original ore (manganese) or the specific metallurgical smelting processes (sulfur, phosphorus). Theoretically, steel smelted without copper, phosphorus and sulfur would have the same mechanical properties. Alloying has as its ultimate goal precisely the improvement of certain technical characteristics of steel. At the same time, phosphorus and sulfur are clearly classified as harmful but inevitable impurities. The presence of copper increases ductility, but promotes the adhesion of the surface of a metal having an excessive (more than 0.3%) concentration of copper onto the surface of an adjacent part. When the structure operates under conditions of intense friction, this is a major drawback.
The presence of a chemical element with a concentration of more than 1% provides grounds for introducing its symbol into the steel grade. In addition to the aforementioned 65G steel, aluminum (present, in particular, in O8Yu steel) also receives a similar honor. In this case, aluminum is introduced into ordinary O8 structural steel in order to deoxidize it, and the fact that its ductility slightly increases is only a fortunate accompanying circumstance. Boriding steel provides it with increased subsequent deformability, therefore even microadditives of boron to the chemical composition of steel are marked accordingly by changing its markings (for example, in 20P steel there is only 0.001...0.005% boron).
In general it is accepted that:
- Steels containing only one element intentionally introduced into the composition;
- Steels containing chemical elements other than carbon and manganese in an amount not exceeding 1%
If the percentage of iron in the melted alloy does not exceed 55%, then such material can no longer be called alloy steel.
Main purposes of alloying
The word "alloying" comes from the German "legieren" (to bind, to connect). The positive effect of alloying components on the properties of steel is associated with ensuring the occurrence of two physical and chemical processes.
Process No. 1
The formation of thermodynamic stable substitution solutions, accompanied by the replacement of part of the iron atoms (ions) in its crystal lattice (ions) of the alloying element. This leads to a distortion of the iron crystal lattice, since the radii of the ions (cations) of alloying elements differ from the radius of the iron cations, which increases the hardness and strength of iron while maintaining its ductility.
Process No. 2
The appearance of strong and practically insoluble chemical compounds in liquid iron between alloying additives introduced into the molten metal and non-metals dissolved in it (oxygen, nitrogen, sulfur, carbon, etc.).
The results of the formation of such compounds are:
- reduction of the residual content of dissolved non-metals in the molten metal, deteriorating its quality;
- reducing the total volume of harmful impurities (dissolved and in the form of non-metallic inclusions) in steel.
And also there is a release (precipitation) from the liquid metal of such small non-metallic inclusions, which serve as crystallization centers and lead to the formation of a fine-grained primary and secondary structure of steel. Due to this, it has better ductility, low anisotropy of properties after rolling, etc. Small non-metallic inclusions released during crystallization tend to accumulate on the surface of growing crystals, reducing the growth rate of the faces, and this, in turn, reduces the grain size of the steel.
Features of alloying
Modern capabilities make it possible to smelt alloyed metals of any composition. Basic principles of the technology under consideration:
- Components are considered alloying only if they are introduced purposefully and the content of each exceeds 1%.
- Sulfur, hydrogen, phosphorus are considered impurities. Boron, nitrogen, silicon, and rarely phosphorus are used as non-metallic inclusions.
- Bulk alloying is the introduction of components into a molten substance as part of metallurgical production. Surface is a method of diffusion saturation of the surface layer with the necessary chemical elements under the influence of high temperatures.
- During the process, additives change the crystal structure of the “daughter” material. They can create penetration or exclusion solutions, and can be placed at the boundaries of metallic and non-metallic structures, creating a mechanical mixture of grains. The degree of solubility of elements in each other plays a big role here.
Drug production
The production process of alloyed tool steels or others takes place in several stages using electric arc furnaces:
- Iron ore is refined.
- Metal melting.
- Adding alloying elements.
The refining process removes unwanted impurities from iron ore, such as sulfur and phosphorus. All this happens in an open melting furnace. The technology of out-of-furnace steel processing is also used. Another technologically necessary process is vacuum smelting, as a result of which arsenic and a number of non-ferrous metal impurities are removed.
An electric arc furnace is already used to melt metal, for which the raw materials are heated to a high temperature of 400-600 °C. Here, iron begins to transform into cast iron, which is characterized by an unstable crystal lattice. But through stabilization, some grade of alloy steel is obtained from it.
This is done as follows. Oxygen enters the working chamber, and when it burns, the atmosphere of the chamber is supplied with carbon. It begins to mix with iron, which leads to the formation of steel.
Then they begin to add various additives to the raw material, depending on the required properties of the metal. The crystal lattice becomes denser, and the result is an alloyed product.
General classification of alloying elements in steels
Metals have a predominant position in the list of alloying elements. The exceptions are silicon and boron.
The presence of alloying elements has a predominant influence on the appearance of the phase diagram of the iron-carbon system, and on the presence/absence of chemical compounds in the final product (nitrides, carbides and more complex components). The latter, in turn, significantly modify the microstructure of steel.
In this regard, steel alloying metals are divided into two groups:
- Metals that increase the region of γ-iron solid solutions (austenitic region on the phase diagram), which leads to increased diversity in the final microstructure of the alloy steel after its strengthening heat treatment). These elements include nickel, manganese, cobalt, copper, and nitrogen.
- Metals and chemical elements, the presence of which narrows the γ-region, but increases the strength of steel. These include chromium and tungsten. vanadium, molybdenum, titanium.
In the process of producing alloy steels, the following patterns in its properties change.
As is known, different elements have different crystal structures (for metals this is face-centered and body-centered). Iron itself has a body-centered lattice.
This is interesting: VGP pipe - decoding, description, advantages and scope of application
When a metal with a similar type of lattice is introduced into steel, the region of existence of an α-solution (ferrite) increases due to a corresponding decrease in the austenitic region. As a result, the microstructure is stabilized, which allows for a wider choice of technological processes for subsequent heat treatment. On the contrary, if the steel contains a metal with a different type of lattice, the austenitic region narrows. Such steel will be more ductile during subsequent machining. Alloying steel with some metals is generally impossible. This occurs if the difference in the atomic diameters of the elements exceeds 15%.
It is for this reason that a metal such as zinc is introduced as an alloying additive only into non-ferrous metals and alloys. Chemical elements that are unable to form stable chemical compounds with carbon, iron and nitrogen during smelting are also of limited use for steel alloying purposes.
The dependence of the characteristics of steel on its saturation with certain chemical elements has not yet been fully studied. This is explained by the fact that with complex doping, each component can interact differently with others, and such changes often cannot be explained naturally. Therefore, questions about the appropriateness of using one or another alloying element are resolved experimentally.
The following provisions are considered proven:
- The efficiency of the process increases with increasing solubility of nitrogen and carbon in the alloying additive and in the main iron;
- The stability of the final properties of steel increases with increasing size of the austenitic zone;
- The quality of steel alloyed with metals and elements with a lower serial number than iron (in D. Mendeleev’s table of chemical elements) is worse than in the opposite case;
- Metals that are more refractory than iron increase the strength of steel in any variant of its further heat treatment.
However, secondary interactions, which strongly depend on the method of steel smelting, can significantly correct these provisions. Therefore, at this stage we can speak with confidence only about the influence of specific alloying elements on the properties of steel.
Nickel
Nickel increases the ductility, toughness, and heat capacity of the alloy, increases its resistance to cracking and corrosion, and improves heat treatment capabilities. In this regard, ferronickel is one of the most common and sought-after ferroalloys in the global metallurgical industry. World standards define five grades of ferronickel containing 20-70% nickel, plus a small amount of carbon (C), sulfur (S), phosphorus (P), silicon (Si), chromium (Cr), copper (Cu).
Nickel-alloyed heat-resistant alloys typically contain 8-25% nickel, and some contain up to 35% or more. However, due to the fact that nickel reduces the hardness of the alloy, it is usually used for alloying not in its pure form, but in combination with iron, chromium, molybdenum, titanium, niobium and other elements. As an example, we can cite alloys of grades 12Х18Н9Т (Fe – about 61%) and 10Х17Н13МЗТ (Fe – about 67%) with a nickel content of 8-9.5% and 12-14%, respectively.
Molybdenum and tungsten
Tungsten and molybdenum have a similar effect on the physical characteristics of steels and alloys, significantly increasing the limit of long-term mechanical strength at temperatures up to 1800°C (in vacuum). It is enough to introduce 0.3-0.5% of these elements into the alloy to significantly enhance its creep resistance, strengthen the interatomic bonds of the crystal lattice, and increase the temperature limit of recrystallization. For the steelmaking and foundry industries, alloying ferroalloys are produced from molybdenum and tungsten with iron: ferromolybdenum (55-60% Mo) and ferrotungsten (65-85% W).
For alloying, relatively small amounts of molybdenum (about 0.2-20%) and tungsten (up to 10-12%) are usually introduced into alloys, since an excess of these elements can increase the brittleness of the alloy when heated. An example of an alloy alloyed with molybdenum and tungsten is heat-resistant low-alloy steel 12Х1МФ (Fe - about 96%) with a Mo content of 0.25-0.35 percent. In the same series is the heat-resistant relaxation-resistant steel 20Kh3MVF (Fe - about 93%) containing Mo 0.35-0.55% and W 0.3-0.5%, as well as the nickel-based alloy KhN57MTVYu (Mo 8.5-10%, W 1.5-2.5%, Fe 8-10%, etc.)
Vanadium
For alloying, relatively small amounts of molybdenum (about 0.2-20%) and tungsten (up to 10-12%) are usually introduced into alloys, since an excess of these elements can increase the brittleness of the alloy when heated. An example of an alloy alloyed with molybdenum and tungsten is heat-resistant low-alloy steel 12Х1МФ (Fe - about 96%) with a Mo content of 0.25-0.35 percent. In the same series is the heat-resistant relaxation-resistant steel 20Kh3MVF (Fe - about 93%) containing Mo 0.35-0.55% and W 0.3-0.5%, as well as the nickel-based alloy KhN57MTVYu (Mo 8.5-10%, W 1.5-2.5%, Fe 8-10%, etc.)
In order to improve the heat resistance characteristics, the composition of alloying elements becomes more complex; molybdenum, chromium, nickel, etc. are often introduced into the alloy along with vanadium. An indicative example of such an alloying technology is a heat-resistant alloy based on iron grade 12Х2МФСС (Fe - about 95%) with a content of V 0.2-0.35%, Mo 0.5-0.7%, Cr 1.6-1, 9%, Ni up to 0.25%, etc. Another example of multi-alloying of an alloy using vanadium is heat-resistant steel 15Kh2M2FBS, including V 0.25-0.4%, Mo 1.2-1.5%, Cr 1.8-2.3%, Ni up to 0. 3%, etc.
Special ferroalloys
All ferroalloys used in the foundry production of heat-resistant alloys are conventionally divided into two groups: the first is ferroalloys for mass use, the second is special ferroalloys. The second group includes compounds of iron with titanium, cobalt, niobium and a number of other elements. Special ferroalloys are used in small proportions of 4–6%, and not only to increase the operating temperature of heat-resistant alloys, but to give them special properties.
For example, ferroniobium is used to alloy heat-resistant chromium-nickel steels, since niobium effectively prevents intergranular corrosion, which destroys grain boundaries and leads to loss of strength of the material. In turn, ferrotitanium is introduced into heat-resistant alloys to enhance the overall anti-corrosion characteristics. In addition, titanium improves the weldability of stainless steels. Alloying heat-resistant alloys with ferrocobalt has a positive effect on their relaxation resistance, especially for chromium steels.
Additional classification
Alloyed structural alloys are suitable for the manufacture of machine parts and mechanisms in the engineering industry - they produce large-sized parts that are hardened and subjected to high tempering. Most alloying additives in steel increase hardenability. The introduction of additives should be sufficient, but not excessive. A high degree of doping can cause:
- decrease in plastic properties;
- development of temper brittleness;
- lowering the threshold of cold brittleness.
The exception is nickel; it shifts the threshold of cold brittleness to the region of low temperatures, so for machines operating in the North, mechanisms are made from nickel-containing steels. Spring alloy steel contains 0.5–0.7% carbon, and chromium, molybdenum and tungsten are added as additives. Such a composition should provide high resistance to small plastic deformations and high fatigue resistance.
Ball bearings - classified as hypereutectoid - carbon is about 1% with additional alloying of the metal with chromium (1.3–1.65%). In heat-resistant bearings, chromium is increased to 5%. Bearings are subject to special requirements for metallurgical cleanliness. The use of refining remelts, vacuum remelting methods, and treatment with synthetic slags make it possible to reduce the proportion and size of non-metallic inclusions, thereby increasing resistance to contact fatigue.
Characteristics of alloy steels
Alloy steel is steel that, in addition to the usual impurities, is also equipped with additional additives that are necessary for it to meet certain chemical and physical requirements.
Ordinary steel consists of iron, carbon and impurities, without which it is impossible to imagine this material. Additional substances are added to alloy steel, which are called alloying substances. They are used to ensure that steel has the properties that are necessary in certain situations.
In most cases, the following are added to iron, impurities and carbon as alloying elements: nickel, niobium, chromium, manganese, silicon, vanadium, tungsten, nitrogen, copper, cobalt. It is also not uncommon for such materials to contain substances such as molybdenum and aluminum. In most cases, titanium is added to add strength to the material.
This is interesting: What is cold welding for metal? Its features, pros and cons
This type of steel has three main categories. The relationship of alloy steel to a particular group is determined by how much steel and impurities it contains, as well as alloy additives.
Types of Alloy Steel
There are three main types of steel with alloying elements:
- Low alloy steel. It is characterized by the fact that it contains about two and a half percent of alloying additional elements.
- Medium alloy steel. This material contains from 2.5 to 10 percent of additional alloying substances.
- High alloy steel. This type includes steel materials, the amount of alloying additives in which exceeds ten percent. The amount of these components in such steel can reach fifty percent.
Purpose of alloy steel
Alloy steel is widely used in modern industry. It has a high level of strength, which allows it to be used to manufacture equipment for cutting and chopping rolled metal of various types.
According to their purpose, alloy steels can be represented by a large number of groups.
The main ones are:
- structural alloy steel,
- tool alloy steel,
- alloy steel with special chemical and physical properties.
The characteristics of alloy steels can be varied. They acquire them due to the ratio of the basic elements. Steels of this type are in any case more durable and resistant to corrosion.
Alloying process
The main way to alloy steel is the method of volumetric metallurgical alloying. It consists of fusing the main element with alloying elements in various types of furnaces (induction, vacuum-arc, crucible, converters, arc, plasma, etc.). With this method, a significant loss of active substances (manganese, chromium, molybdenum, etc.) is possible.
There are also:
- mechanical alloying;
- recovery;
- electrolysis;
- plasmachemical reaction.
Mechanical alloying is performed in attritors - drums, in the center of which there is a shaft with cams. Powdered components are placed in them to obtain the desired alloy. During rotation, the cams “hit” the mixture, and the alloying additives are “driven” into the base.
In co-reduction, the oxides of the alloy elements are mixed with a reducing agent, for example, calcium hydride (CaH2) and heated. The reaction of reduction of oxides to metals occurs, and the diffusion process occurs simultaneously, leveling the composition of the alloy. The resulting calcium oxide (CaO) is washed with water, and the alloy (in powder form) goes into the next processing. Metallothermic reduction involves the use of metals (magnesium, calcium, aluminum, etc.) as reducing agents.
With the help of surface alloying, the surface of the product is given special properties. A certain element or alloy is applied to the top layer in the form of a small layer, then it is exposed to energy (laser radiation, plasma, high-frequency current, etc.) - the surface is melted, and a new alloy is formed on it.
Low-alloy steel, high-quality structural
Regulatory document: high-quality structural low-alloy steel is manufactured in accordance with GOST 19281-89.
Low-alloy steel - alloy steel with a total mass of alloying elements less than 2.5% of the total mass of steel.
Low alloy steel grades
Steel grades: 09G2, 09G2S, 0KHSND, 17G1S, 16G2AF, 10KHNDP, 15KHNDP, 0KHSND, 15KHSND, etc.
Low-alloy steel grades 10KhNDP, 15KhNDP, 0KhSND, 15KhSND are atmospherically corrosion-resistant (AKS).
Substitutes for some grades of steel:
- 09G2S - 09G2, 09G2DT, 09G2T, 10G2S;
- 10HSND - 16GAF.
Application of low alloy steel
Low-alloy steel is used for the manufacture of railway, metro and tram car bodies, load-bearing structures of locomotives, agricultural and other field machines and engineering structures operating under conditions of variable dynamic loads and seasonal and daily heat changes.
Weldability: low-alloy steel can be welded without restrictions.
Properties and purpose of alloy steels
The presence of alloying elements and subsequent processing provide steel with a number of unique physical and chemical properties:
- Heat resistance
- Wear resistance
- Plastic
- Corrosion resistance
- Durability and many others.
Thanks to this, alloy steels are actively used to perform various technical tasks in almost all industrial fields: medical equipment and instruments, containers and equipment in the food industry, shafts, washers, gearboxes, components, structural elements in construction and mechanical engineering, etc.
Rating: 9.80/10 — 46 votes
Tool alloy steels
Tool alloy steels
This type of low-carbon iron has other priority parameters, focused on high levels of hardness and wear resistance. Both characteristics improve with increasing carbon concentration in the metal.
The primary issue affecting alloy steels is the use of this type of metal. The area of use, as stated earlier, corresponds to the name of the category. Such steel is a material for the production of three main groups of tools:
- cutting;
- measuring;
- stamps.
The first category combines cutters, cutters, and cutters. This also includes a class of high-speed steel, characterized by red resistance, as well as preservation of cutting characteristics when heated to a temperature of 700 0C. Another distinctive feature of high-speed steel is the metal processing speed, which is five times higher than that of conventional tool grades. High-speed grades are marked with the letter “P”, where subsequent numbers indicate the percentage of tungsten.
The document describing tool alloy steels is GOST 5950 – 73. This type has improved heat resistance, the range of values of this parameter is transferred to the range 250 – 300 0C. An increase in this characteristic affects the cutting speed, increasing its value by 20 - 40%.
Considering how alloying elements influence the properties of steel, we will focus on several elements.
Silicon, grade – 9ХС. The introduction of the element into the composition of tool steel increases its hardenability to 40 mm. An additional effect is associated with improved martensite resistance during tempering. However, the element also brings negative nuances to the alloyed metal. Steels containing silicon are difficult to cut.
Alloy structural steel products
Manganese, grades – KhVG, 9KhVSG. Alloying with this metal leads to a decrease in tool deformation during the hardening process. This type of alloying is most effective for broaches - tools with a large ratio of length to cross-sectional diameter.
Description of the term - what is alloy steel
Physical properties, such as strength, ductility, fragility, can be increased or decreased several times. Changing the crystal lattice of materials is actively used in metallurgy, as well as in the production of numerous parts and housings for automotive, machine, machine tool and other production, as well as for the creation of building structures and tools. The scope of application is so wide that the alloy began to be produced in large quantities; it is gradually replacing the share of manufactured iron and ordinary steel substances.
Based on the information provided, steel alloying is a metallurgical smelting process during which impurity materials are added to the composition. There are two types of operations:
- Volumetric – when the components fall into the deep structure. Chromium, nickel, etc. are introduced into the melt or charge.
- Surface - during it, diffusion or other spraying occurs, that is, only the top layer is covered.
The process has come into use relatively recently. Experiments began for the first time in 1882. And from the very first sample, the researchers discovered that along with the improvement in physical properties, the degree of machinability was significantly reduced. In simple words, the material simply became difficult to work with. Of course, by now all the additional effects of alloying have been studied, so special GOST standards have been drawn up for different metalworking methods.
Alloyed scrap metal
The review of the alloy scrap market concerns not only steel, but also cast iron. Indeed, the share of advertisements for the purchase of alloyed cast iron scrap is not particularly inferior to the demand for secondary low-carbon iron. Acceptance of alloyed scrap is carried out by almost all points working with ferrous metal, but at a significantly higher cost.
It is worth understanding: for scrap metal collection points there is no such division by alloy steel (as in the directory) - for them there is black scrap, stainless steel scrap and high-speed cutter scrap. If everything is clear with stainless steel and quick-cutting steel, then black scrap can include steels such as: 09G2s and other grades that are in demand in this particular region. Some enterprises specifically purchase scrap steel from 09g2s.
Naturally, taking into account the specifics of alloy waste and alloy steel scrap, the price of such scrap per kilogram is determined by the inclusion of certain metals - alloying elements. For example, recycled steel with a nickel content of more than 9.3% can be accepted at up to 60 rubles per kg, while a lower concentration of Ni equates the waste to ordinary black steel scrap - 11,000 per ton.
Alloy scrap
Of particular value are high-speed grades, the value of which even in the form of scrap metal is significantly higher. However, many receivers divide the waste of high-speed cutters into two categories. The first group includes grades R6M5, R18, used for processing metals, including alloyed structural steels. The second includes grades P9 and P12, used for working on stone and less hard materials - see the article high-speed steel scrap.
Thus, the cost of alloy steel scrap is determined mainly by a couple of parameters: the content and type of additive, as well as the quality of the steel itself. On the other hand, high-speed cutter scrap, unlike other steel waste, can be used for business purposes. Many tools, even after completing their service life, remain attractive for further use. The scope of their application may include both the household sector and small private enterprises.
Ball bearing high-quality structural steel GOST 801-78
Regulatory document: high-quality structural alloy ball bearing steel is manufactured in accordance with GOST 801-78.
Classification of ball bearing steel
Depending on the surface quality requirements and depending on further processing:
- for cold machining - OX;
- for hot pressure treatment - exhaust gas;
- for cold heading - ХВ;
- for cold stamping - ХШ.
By shape, size and maximum deviations:
- hot rolled steel circle 40x - GOST 2590-88;
- hot-rolled square - GOST 2591-88;
- square blank - according to current regulatory documents;
- hot rolled strip - GOST 103-76;
- calibrated wheel quality h11 with additional dimensions - GOST 7417-75;
- circle with special surface finishing of quality h11 groups B and G - GOST 14955-77.
According to the condition of the material:
- without heat treatment;
- heat treated.
Grades of ball bearing structural steel
Steel grades: ShKh15, ShKh4, ShKh15 SG, ShKh20 SG.
Designation of steel grades: Ш - bearing, X - alloyed with chromium, number - chromium content, SG - alloyed with silicon and manganese. For example, ball bearing steel and spring steel ShKh15.
Substitutes for some grades of steel:
- ShKh15 - ShKh9, ShKh12, ShKh15 SG;
- ШХ15 SG - ХВГ, ШХ15, ХС, ХВСГ.
Application of ball bearing steel
Manufacturing of parts operating under the influence of concentrated and alternating stresses arising in the contact zone of balls and rollers with the running tracks of rolling bearing rings. ShH15 is especially popular.
Weldability: welded using the KTS method.
Carbon steels
Carbon, while increasing hardness, also makes the alloy more brittle. The percentage content of the element is reflected in the marking - you can use it to determine what material is in front of you. Please note that the first two digits reflect the presence of hundredths of a percent of the element, one – tenths. If the carbon content is up to 0.25%, then the steel is low-carbon, and therefore inexpensive, easy to weld. If from 0.3 to 0.55%, then the alloy is medium carbon; these are actively used in mechanical engineering. The amount of the element in the range of 0.6-2% shows that the material is high-carbon, therefore its weldability and fluidity are low, but its hardness is high.
The structure of low-carbon alloys provides plasticity, but relatively low strength of the material. An increase in carbon content leads to a loss of ductility, but significantly increases strength. Thus, high-carbon steel is a very hard, durable alloy, for which they try to avoid the use of welding whenever possible. Wire, bearings, springs, and stamped parts are produced from it.
This is interesting: Characteristics of food grade stainless steel. What grades of stainless steel are food grade?
Classification
The group of low-alloy steels includes steels that differ in:
- Chemical composition . For alloying, various elements are used, often not scarce - nickel, molybdenum, chromium, aluminum, silicon.
- Heat treatment . The types of heat treatment used are quenching + tempering, normalizing + tempering, various types of annealing.
- Weldability . Grades with a low percentage of carbon have good weldability.
List of the most popular grades of low-alloy steels:
- 09G2S and alternative options - 09G2, 09G2T, 09G2DT, 10G2S;
- 17G1S;
- 10HSND and alternative – 16GAF.
The group of atmospheric-corrosion-resistant steel alloys (ACS) includes 10KhNDP, 15KhNDP, 15KhNDP, 15KhSND, 0KhSND.
Why label?
Alloys are marked according to GOST. The brand indicates their purpose, basis, and the presence of impurities. For example, they can be instrumental (used to make working parts of various tools), structural (used to create metal structures, car bodies). Additional letters may denote materials that have special physical properties (magnetic, heat-resistant, corrosion-resistant).
The quality of the alloy is determined by the percentage of additives in the composition. For example, the content of phosphorus and sulfur should be minimal. The components are indicated by capitalizing the element letters.
Welding
To connect parts made of low-alloy steel using welding, you need to take into account several nuances:
- Make vertical, ceiling seams.
- The welding rod must have a cross-section of at least 4 mm.
- To reduce the cooling rate of the metal, it is necessary to make butt or side welds.
- When welding workpieces with a thickness not exceeding 6 mm, only one pass is required.
- To give the connection high ductility, you need to use E42A electrodes.
- If the metal contains a small amount of carbon, it is necessary to use electrodes coated with fluorine and calcium.
To carry out welding work, it is necessary to use a special additive Sv-10G2.
Low-alloy steels have increased technical parameters due to the addition of additional components to the composition. They are used in those areas of industry where it is necessary to use parts and metal structures of high strength and wear resistance. To connect individual parts, you need to take into account a number of nuances of using welding equipment.
Decoding
Steel is marked according to certain rules. The first numbers indicate the carbon content. For example, 5 indicates 0.05%. If there is no number at the beginning, the amount of carbon is up to 1%.
The letters that follow the first numbers indicate the name of the additional components.
There are different types of steels that differ in technical characteristics and properties. They depend on the presence of additional impurities contained in the composition. To avoid confusion among numerous products made from different types of materials, special markings are used.
CIS countries standards
When designating alloy structural steel, the percentage of carbon mass fraction is marked with the first two digits without using a letter designation. Next, alloying components and their share in the alloy in average equivalent are indicated in decreasing order. The letter designations of chemical elements are indicated in Table 1. Alloying additives, the amount of which is less than 1.0%, are indicated only in the deciphered nomenclature, since the designation would then take on a very cumbersome form.
Given the extensive assortment, the steel grade may also include additional symbols, since the designation would then take on a very cumbersome form that more broadly describes the properties or features: A - automatic, E - magnetic, F - stainless, P - cutting, X - chromium , Ш - ball bearings, E - electrical, I - chromium-nickel. Labeling may also involve exceptions to the general designation rules. So, depending on the chemical composition, structural alloys are divided into high-quality and high-quality. For example, at the end of the marking, the letter “A” indicates that the alloy is especially pure in terms of phosphorus and sulfur content, and the letter “W” classifies them as high-quality.
Marking of alloy steels for river and sea shipbuilding is often carried out in accordance with GOST 5521-86 and the requirements of the International Association of Classification Societies. This means that such alloys are classified into categories A, B, D and E, taking into account their yield strength, strength, brittleness and impact resistance.
European standards
EN 10027 defines the designation procedure for all steels. Alloy alloys are marked 1.20ХХ - 1.89ХХ, where the first digit determines that the material belongs to steel, the second and third digits determine the number of the steel group, and the last two determine the serial number of the alloy in this group. For example, the category of tool steels is identified as 1.20ХХ - 1.28ХХ, and stainless steels as 1.40ХХ - 1.45ХХ.
North American ASTM/ASME and AISI standards
The USA has the most extensive steel marking system. For example, ASTM marking implies the designation of the main chemical elements, tensile strength and shape of the rolled product. The AISI system uses 4 digits, where the first two indicate the group number, the next two indicate the percentage of carbon. Letter symbols indicate the presence of the corresponding additives.
Compound
Before you start understanding the properties, you need to know the composition of low-alloy steels. The amount of alloying additives should not exceed 5% (some sources indicate the maximum amount of additional components - up to 2.5%). Carbon is not considered an alloying component.
The most popular, inexpensive additional additives include:
- Vanadium is responsible for the uniform structure.
- Molybdenum - increases the resistance of the compound to high temperatures.
- Niobium - increases strength.
- Tungsten - increases heat resistance.
- Titanium - increases wear resistance.
- Nickel, silicon - increase shock resistance and current resistance.
Examples
To learn how to decipher symbols, you need to consider several labeling options:
- U8GA - contains 0.8% carbon.
- St3sp5 is a structural metal that is not alloyed. Often used for the manufacture of metal structures.
- 30ХГСА - contains up to 0.3% carbon. Additional components - silicon, manganese, chromium. The letter A indicates high quality material.
- R6M5F2K8 - high-speed steel. The composition contains about 8% cobalt, 5% molybdenum, 2% vanadium.
- HVG - consists of manganese, chromium, tungsten, the amount of which does not exceed 1%.
Instrumental types
Alloyed tool steel
Alloyed tool steel is intended for the production of metal-cutting tools operated at high cutting speeds and for the manufacture of stamping tools.
High-speed steels are capable of maintaining high hardness and wear resistance of the cutting edge of the tool. Molybdenum, vanadium, tungsten, chromium and cobalt are added to this steel.
Cold work die steels containing 1.0–2.0% carbon have wear resistance and toughness. They are alloyed with chromium up to 12%, vanadium, tungsten, and molybdenum.
Die steels for hot deformation contain carbon in the range of 0.3–0.5%, have high heat resistance, impact toughness, and thermal fatigue resistance. Tungsten, molybdenum, and vanadium are introduced as additives.
Welding alloy steels: features
Alloy alloys have good ductility, so complex structures can be made from them by welding. Due to the different content of additives, each type of alloyed products has its own characteristics.
Welding alloy steels
Alloy alloys have good ductility, so complex structures can be made from them by welding. Due to the different content of additives, each type of alloyed products has its own characteristics.
Welding low alloy steels
The peculiarity of welded joints of low-alloy steels is their high resistance to cold cracks and brittle fracture. But, such properties of the connecting seam can only be achieved with proper welding.
If the preheating process is disrupted or the weld is subjected to too rapid cooling, the metal may receive microscopic damage at the joints, which will significantly reduce the strength of the entire structure.
Low-alloy steels 10G2SD, as well as 14KhGS and 15KhSND are welded using a direct current machine with reverse polarity. Electrodes for welding must have a calcium fluoride coating.
The amount of welding current must exactly match the type of electrode, the thickness of the metal and the type of alloy.
Failure to comply with this requirement will also affect the quality of the weld and, as a result, the strength of the manufactured structure.
Welding of low-alloy steel must be carried out without interruption so that the entire seam is made without a metal temperature of at least 200 degrees. The average welding speed is 20 m/h, with a voltage of 40 V and a current of 80 A.
Welding of medium alloy steels
When manufacturing structures from medium-alloy steels, it is necessary to use welding materials in which the content of alloying elements should be less than in the material being welded.
Only by using such materials can a seam with high resistance to deformation be achieved. If, in the manufacture of products from medium-alloy steels, the sheet thickness does not exceed 5 mm, then high quality joints can be achieved using argon arc welding.
If gas welding is used to connect parts, then acetylene mixed with oxygen should be used as a combustion source.
Welding of high alloy steels
If high-alloy steel is used for the production of metal parts, then welding equipment with minimal thermal entrainment of the material should be used. This is necessary to reduce the likelihood of metal warping during welding, due to the high content of various impurities in the metal composition.
Electric welding of high-alloy alloys is carried out using calcium fluoride coated electrodes. In this case, it is possible to achieve high levels of mechanical and chemical strength of the weld.
The use of gas welding in the manufacture of structures made of high-alloy steels is undesirable. In exceptional cases, it is possible to use gas welding to connect heat-resistant high-alloy steel sheets with a thickness of no more than 2 mm.
Sources
- https://www.syl.ru/article/365687/chto-takoe-legirovannaya-stal—sostav-svoystva-marki-gost-naznachenie-obrabotka
- https://FB.ru/article/275232/legirovannyie-metallyi-opisanie-spisok-i-osobennosti-primeneniya
- https://www.m-deer.ru/tehnologiya/legirovanie-stalej.html
- https://www.metotech.ru/art_garsplavy_2.htm
- https://intehstroy-spb.ru/spravochnik/legirovannye-stali-klassifikaciya-i-markirovka.html
- https://xlom.ru/spravochnik/legirovannyj-stali-vidy-xarakteristika-legirovannyj-metallolom
- https://msmetall.ru/metalloobrabotka/klassifikaciya-legirovannyh-stalej.html
- https://metalloy.ru/stal/markirovka-legirovannoy-stali
- https://metinvest-smc.com/ru/articles/legirovannaya-stal-osobennosti-klassifikatsiya-i-kharakteristiki/
- https://plavitmetall.ru/obrabotka/legirovannaya-stal-primenenie.html
- https://regionvtormet.ru/stanki-i-oborudovanie/harakternye-osobennosti-legirovannoj-stali-i-sfera-ee-primeneniya.html
Alloy (stainless) steel: steel grades and their characteristics
Stainless steel is a type of alloy steel whose corrosion resistance is achieved by containing at least 10.5% chromium and having a low carbon content. In the presence of oxygen, chromium oxide is formed, which creates an inert film on the surface of the steel, protecting the entire product from adverse influences. Alloy (stainless) steel has high characteristics of corrosion resistance, resistance to aggressive environments, ductility and strength. It is used to produce a wide variety of products - from medical instruments to large building structures.
“Stainless steel” is a general name for steels with increased resistance to corrosion, but not every grade of stainless steel demonstrates equal resistance of the chromium oxide film to mechanical and chemical damage. For different tasks, by combining alloying elements and their composition, special grades of stainless steel and alloys have been developed. The classification of stainless steels differs slightly depending on the country, but is generally similar and based on the same principles. Based on the chemical composition, properties and internal structure of the metal, the following types are distinguished:
- Ferritic. This group of steels is characterized by a high chromium content, usually more than 20%. Therefore, this type is sometimes called chromium. This chemical composition contributes to high resistance to aggressive external environments. Alloys of this group have magnetic properties. Ferritic steels are relatively cheap and are widely used in industry, second only to austenitic steels.
- Austenitic. A group of anti-corrosion alloys that are characterized by a high content of chromium and nickel. Due to this, they are distinguished by increased strength and flexibility in comparison with analogues. Also easy to weld and resistant to corrosion. Most widely used in industry. They belong to non-magnetic metals.
- Martensitic. A special type of stainless alloy. It is characterized by increased strength and wear resistance. They are not exposed to high temperatures, and at the same time contain a minimal part of harmful components that do not emit vapors during intense heating. This group includes heat-resistant, corrosion-resistant steel.
- Combined. A special type of steel that combines the properties of the above groups. Such innovative steels are developed individually depending on the properties required by the customer. Today, austenitic-ferritic and austenitic-martensitic steels are distinguished.
The Russian metal market offers various grades of stainless steel for use in the oil and gas, chemical, food, energy and other industries and is represented by several large “players”, such as the FERRIT Group of Companies, the metal trading company Continental, the Ileko group of companies, Globus-Steel, which Initiative LLC can recommend based on supply experience, and many small companies. Below are the stainless steel grades most commonly used in chemical engineering (Russian steel grades and their foreign analogues :
08Х13, (0Х13 EI496, AISI 409) - this steel successfully combines several important parameters, such as high strength and good mechanical properties, increased resistance to climatic corrosion, ease of processing, ductility, the ability to use several options for processing - drawing, stamping, perforation. At the same time, this steel has serious restrictions on its use - it is used to produce products that do not experience shock loads during operation, as well as exposure to low temperatures, for example, internal structures of columns.
12X15G9ND (AISI 201) - provides a significantly more favorable price-quality ratio compared to classic grades of stainless steel with similar properties, since expensive nickel is partially replaced with manganese and nitrogen. The advantageously balanced chemical composition makes the characteristics of AISI 201 stainless steel not inferior to AISI 304 and AISI 321 and is gradually catching up with them in popularity. At T>1260°C it is easy to forge and upset. Forgings can be cooled in air. It is also easy to process when cold. Very strong and ductile during deep drawing, bending, stamping and upsetting. It is strain-hardened during cold working, similar to steels of type 12Х18Н10Т. It can be easily welded by arc welding using a protective atmosphere. This steel has found its application in the medical and food industries. It is also used in the manufacture of round and profile pipes, which are required to create railings, handrails and fences.
08Х18Н10, 08Х18Н9 (AISI 304) - the most common and in demand in all industries, this stainless steel has gained fame as “food grade”, since its chemical composition and properties make it most suitable for use in the food industry. It is characterized by high strength, elasticity, is easy to weld, and shows high corrosion resistance characteristics in aggressive environments. This steel is often chosen for the chemical, pharmaceutical, petroleum and textile industries.
10Х17Н13М2 (AISI 316) is an improved version of AISI 304 due to the addition of molybdenum, which increases anti-corrosion resistance and the ability to maintain properties in aggressive acidic environments, as well as at high temperatures. Finds application in the chemical, oil and gas and shipbuilding industries.
10Х17Н13М2Т, 10Х17Н13М3Т (AISI 316Ti) - this grade of stainless steel, compared to AISI 316, is additionally alloyed with a small amount of titanium, which increases the strength of the material, making it resistant to high temperatures, as well as to chlorine ions. Used in welded structures, for the manufacture of gas turbine blades, in the food and chemical industries.
12-08Х18Н10Т (AISI 321) Stainless steel, the characteristics of which are determined by the presence of titanium in the chemical composition. Easily weldable, resistant to temperatures up to 800°C. Widely in demand for the manufacture of seamless pipes, as well as pipeline fittings - flanges, tees, bends and reducers.
06ХН28МДТ (.0Х23Н28М3Д3Т EI943, AISI 904L) - an alloy of this grade is optimally suited for creating welded structures that will subsequently be used at temperatures up to 80 ° C in sulfuric acid of various concentrations, with the exception of 55% acetic and phosphoric acids.
20X23H18 (AISI 310S) - heat-resistant stainless steel is easy to shape and has good weldability, which makes it widely used in production. AISI 310S also has oxidation resistance properties due to its composition and increased heat resistance, as it can withstand high temperatures in various environments. Various equipment for the chemical and oil industries are made from it: installations for methane conversion, pyrolysis, gas pipelines, combustion chambers, as well as for the production of heating elements.
12X17 (AISI 430) is a stainless steel with a high percentage of chromium and low carbon, which contributes to high strength and at the same time ductility. It is an economical option for a corrosion-resistant material, ideal for stamping, deformation and perforation, bends and welds well. This steel retains its properties in corrosive and sulfur-containing environments and is resistant to sudden temperature changes. It is used in the oil and gas industry, as well as as a decorative material for finishing buildings and premises.
Footnotes
- Alloy steels are designated by listing the alloying elements, designated by letters, with the approximate percentage of the alloying element indicated after each letter. The letter designations, in particular, are as follows: X - chromium, N - nickel, A - nitrogen, E - selenium, T - titanium, P - phosphorus, B - niobium, V - tungsten, F - vanadium, M - molybdenum, G - manganese, D - copper, P - boron, C - silicon, Y - aluminum, etc. So X18N10 means that this steel contains about 18% chromium and about 10% nickel.
- If the alloying element is one percent or less, only the letter designating it is written, without indicating the percentage of its content after it.