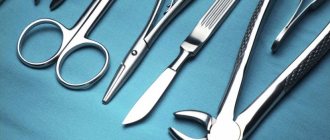
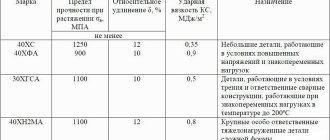
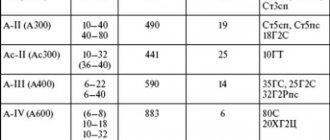
Causes of corrosion during welding
The appearance of corrosion primarily at welded joints is due to two reasons:
- the breaking of primary intercrystalline bonds and the formation of new ones, different in their mechanical properties from the previous ones, as well as the appearance of stresses in the metal structure.
- changes in the chemical composition, the appearance of oxides (endogenous non-metallic inclusions), which are stress concentrators in the structure;
- the formation of a galvanic couple due to changes in chemistry. composition of the weld.
The influence of the above changes increases in proportion to the degree and intensity, number and size. The rate of the oxidation reaction is additionally determined by operating conditions: climatic component, physicochemical influence (working environment).
Types of weld corrosion
At the moment, we have sufficiently studied what types of corrosion destruction of welded joints exist and, depending on the nature of their action, appropriate protective measures are applied to them.
Based on the nature of destruction, corrosion is divided into 3 types:
- Solid
Predominant mainly in carbon unalloyed or low alloy steels with a homogeneous structure. A seam, regardless of the steel grade, is always destroyed more intensively than an integral plane.
Appearance of continuous corrosion
- Spot or local
Steel that is heterogeneous in its chemical composition is predisposed to this type of destruction. Such corrosion develops in weakly rusting steels of the X12MF type or in welds depleted in chromium. It is also determined by operating conditions.
Local metal corrosion
- Intercrystalline or knife
The most dangerous type of corrosion. Fracture occurs along the grain boundaries of the metal throughout its thickness. Austenitic (stainless) steels that are subjected to prolonged heating above 600 ºC, including welding, are susceptible to this “disease”.
Intergranular corrosion of metal
Galvanic couple also contributes to this type of destruction: after melting, the welded joint changes its chemical composition and, when exposed to an electrolyte, which can even be water, is depleted first. This type of corrosion “works” can simultaneously work on a large area of metal, which is destroyed even under a small load.
CORROSION RESISTANCE AND STRENGTH OF WELDED JOINTS
types and FEATURES of corrosive damage to welded joints
The types and characteristics of corrosion damage to metal structures, including welded ones, are determined by the properties of the material, the stress state in the structure, the properties of the corrosive environment and the conditions of interaction of the metal with the environment (temperature, time, contact conditions, pressure, etc.). The mechanism of corrosion destruction of welded joints does not differ from the mechanism of destruction of the base metal. The features that determine (in contrast to the base metal) the causes, nature, kinetics and mechanism of destruction of welded joints depend on the physical and chemical effects of welding, causing an unfavorable change and heterogeneity in the properties of the metal and the stress state, resulting in an increase in the negative impact of the environment.
A welded joint is characterized by: a) structural-chemical macro- and micro-inhomogeneity (base metal, cast weld metal, transition structural zones of influence; grains, grain boundaries, inclusion phases, etc. within each zone); b) heterogeneity of the stress state of its own (residual stresses and plastic deformation) and from external load; c) geometric heterogeneity (technological and structural concentrators). These main types of heterogeneity determine the mechanical, physical and electrochemical macro- and micro-heterogeneity of welded joints (Fig. 1) and the features of corrosion destruction of welded joints (Fig. 2).
Based on the mechanism, corrosion is distinguished between chemical and electrochemical. Chemical corrosion is the process of interaction of a metal with an aggressive component of the environment (dry gases, non-electrolytes) according to the reaction Me -+- x -*■ Me x. Electrochemical corrosion is the process of spontaneous destruction of a metal as a result of electrochemical reactions, the speed of which determines the rate of corrosion: a) anodic reaction - the transition of metal ions Me+ into solution, leaving an equivalent number n electrons e in the metal; Me°—ne-+ MeL+, which determines material losses during corrosion; b) cathodic reaction—the process of reduction of oxidative components of the environment D (H+, O2, Cl2, Br2, NOJ, etc.) due to the addition of excess electrons appearing in the metal (depolarization process D + ne -> (Dne); c) process the flow of electrons through the metal and the corresponding movement of cations (+) and anions (-) in the solution, i.e., corrosion current [1]. In the vast majority of environments, the corrosion process is electrochemical.
Based on the type of corrosion damage, a distinction is made between general (continuous) corrosion (uniform, uneven, selective); local (localized in the form of ulcers, points, under the surface, intercrystalline); cracking under the influence of static and cyclic loads.
Features of general electrochemical corrosion of welded joints are associated with electrochemical heterogeneity of two types: a) macroheterogeneity, caused by differences in the chemical composition and structure in different zones of the joint; b) microheterogeneity caused by structural and chemical heterogeneity within each zone (Fig. 3). Therefore, in terms of corrosion, the welded joint is a complex multielectrode short-circuited electrochemical system, characterized by macro
the electrodes of which are the seam, the heat-affected zone with a series of transition structures, and the base metal (Table 1).
Macroheterogeneity is assessed by the value of the average electrode potentials f of each zone, microheterogeneity - by the value of local electric
| Rice. 1. Heterogeneity of welded joints in cross section: a — maximum temperatures; 6 — welded joint diagrams; c - hardness HV, HB, grain size and density of the oxide film; g - mechanical properties during bending; P—bearing load, kgf; a is the bending angle; d — electrode <р and thermoelectric E potentials, mV; e - residual plastic longitudinal eXy and transverse deformations; p is the approximate dislocation density; g - residual longitudinal axu and transverse stresses oyy, kgf/mm2: 1 - StZsp; 2 - I2X18H10T; 3 - VT1-1; 4 - AMgb, 6=3 mm, argon arc welding with tungsten electrode |
trodny potentials f; within each zone. An indicator of the macroelectrochemical heterogeneity of a welded joint is the difference in initial potentials Aph between zones. An indicator of the microinhomogeneity of each zone is the scatter of the initial local potentials Af{. = Fitah - F/type within each zone.
The susceptibility of the welded joint as a whole or any of its zones to the effects of corrosive environments and the corrosion rate depends on the general and local electrode potentials and polarizability. The more negative the potentials and the greater their difference, the greater the corrosion rate, as a rule.
Rice. 2. Types
Depending on the values of Df and Df/, the following characteristic cases of general corrosion of welded joints are possible: a) Df > 0, Df/ -> 0, - corrosion but predominantly by the macroheterogeneous electrochemical mechanism. Determination of chp and df" allows one to judge, to a first approximation, the instability of various zones
Rice. 3. Schemes of welded joint corrosion:
/ is the characteristic distribution of initial macropotentials in the welded joint; Fsh is the electrode potential of the weld;
F3 t. in ~ electrode potential of the heat-affected zone; fm is the electrode potential of the base metal; Fust is the established stationary potential of the polarized system: seam - heat-affected zone (HAZ) - base metal; II - macroelectrochemical corrosion, macropair base metal - heat-affected zone, macropair heat-affected zone - weld, macropair base metal - weld with a heat-affected zone; III - corrosion of self-dissolution of each zone: / - heterogeneous
ny mechanism of electrochemical corrosion; 2 - homogeneous mechanism of electrochemical corrosion; 3 - chemical corrosion of the entire connection as a whole; b) Df -> 0, Df/ >0 - corrosion by a predominantly microelectrochemical mechanism; c) Df > 0, Df/ >0 - mixed corrosion mechanism.
General (solid) electrochemical corrosion is characteristic of welded structures made of carbon and low-alloy steels in most natural environments (atmosphere, aquatic environments, soil).
Local selective types of corrosion are typical for welded joints of high-alloy steels and non-ferrous metals in environments in which the metal
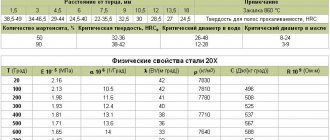
| I. Electrode potentials of various zones of welded joints in 3% NaCl Electrode potential, mV | ||||
| Material | Welding method | Basic metal | Welded the seam | Heat Affected Zone |
| Low carbon steels low alloy: 09G2S | Manual arc, electrode | —460 | -540 | -480 |
| 17G2S1 | type E55 | -500 | —540 | -550 |
| 17G2SF | -455 | -5-10 | -485 | |
| Corrosion resistant become: 12Х18Н10Т | Argon-arc | + 137 | + 108 | +75 |
| 10X14AG15 | Manual without additive, | -170 | — 163 | —250 |
| OZL8 electrodes | ||||
| Aluminum alloys | ||||
| AMgb | Argon-arc without | —492 | —514 | -567 |
| AM g 62 | cages | -680 | -700 | -880 |
| Technical titanium | Argon-arc without | -125 | -180 | —140 |
| you Zirconium | cages | —455 | -393 | —446 |
| Tantalum | Electron beam | —180 | -240 | -212 |
| Niobium | —241 | —255 | -280 |
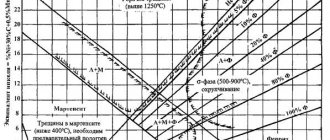
is in the passive and passive-active region. Intercrystalline corrosion associated with structural changes in steels is typical when they are exposed to heating to critical temperatures of 450–900 ° C for austenitic steels and above 900 ° C for high-chromium ferritic steels. An example is intergranular corrosion of welded joints of austenitic chromium-nickel steels, discussed in detail in [6]. Destruction develops in three zones: in the base metal, heated during welding to 500-900 ° C, in the weld and in the base metal near the fusion line in a narrow zone, heated to temperatures above 1200-1250 ° C (knife corrosion). The predominant destruction of grain boundaries is due to the electrochemical heterogeneity of the metal, which occurs under temperature-time conditions specific to each alloy due to the release of excess phases. If excess phases form extended chains along grain boundaries, then corrosion destruction takes on an extremely dangerous intergranular character. The most characteristic excess phases of corrosion-resistant steels, almost always present in them, are carbides. Depending on the chemical composition of the steel and the conditions of thermal action on them, carbide phases [2] of the following types are formed: MS (Mє Ti, Nb, W, Zr, Ta); M2C (MeW, Mo); M3C (iron based); MfCgnM^Q (chromium based), M^M^C (M'eFe, Ni, Co, Si; M'geW, Mo, Ta, V, Cr, Nb); often n + t = 6; M12S. Along with carbide phases, the formation of other phases is possible, which are compounds of steel components with non-metals (nitrides, carbonitrides, sulfides, borides, etc.), as well as inter-metallic phases (o, l), a number of Laves-type phases (Fe2Mo, FeaW, Fe2Nb), strengthening phases [M3Ti, M3A1), etc.
A schematic diagram of the influence of temperature-time conditions on the precipitation of carbides and intercrystalline corrosion is shown in Fig. 4. Temperatures of 850 ° C are favorable for the preferential precipitation of M23Sb (curve /). Knife corrosion is associated with the release of a chain of carbides of stabilizing elements (MS) under the influence of high temperatures (> 1200-1250 ° C) along the grain boundaries (heat-affected zone). Since the corrosion rate (boiling 56% HN03) of titanium carbide is approximately 1000 times, and niobium carbide 3-4 times greater than the corrosion rate of steel, selective dissolution of carbide particles located along the grain boundaries occurs under the catalytic action of ■this process to dissolve adjacent areas of steel. Additional thermal exposure in the critical temperature region (application of a second weld) leads to the release of chromium carbides (M23C6), which complicates the mechanism of knife corrosion and increases its speed. In oxidizing environments, acid-resistant chromium-nickel steels stabilized with titanium are less resistant than
stabilized with niobium, which, in turn, are less stable than those with a low carbon content.
Intercrystalline corrosion of aluminum alloys is also associated with the precipitation of excess (intermetallic) phases such as CuA12, Mg2Al3, MgZn2, etc.
Pitting corrosion is typical for passivating metals (chrome, aluminum, chromium-nickel steels, etc.) and occurs as a result of damage in certain areas of the passive film. In welded joints, pitting corrosion is primarily affected by the heat-affected zone.
Corrosion destruction of welded joints
in a stressed state [4]. The stressed state affects the corrosion behavior of the metal due to: a) imparting additional energy to the metal, due to which it is easier for the Me+ ion to leave the lattice of a deformed metal compared to an undeformed one; b) violations under the influence of deformation of the continuity and protective properties of surface films; c) an increase in the degree of heterogeneity associated with the appearance of crystal lattice defects and new anodic phases under the influence of deformations.
The corrosion rate q changes relatively little under the influence of elastic stresses in neutral and alkaline media, but can noticeably (2-3 times) increase in acidic media in proportion to the stresses: q = q0 - f - a o, where is the speed without tensile stresses; a is the proportionality coefficient. While having a slight effect on general corrosion, stresses intensify local types of corrosion, the most dangerous of which are cracking caused by static loads and corrosion fatigue under cyclic loading.
Corrosion destruction in a stressed state is determined by corrosion, mechanical and sorption processes, as well as accompanying processes (cavitation, radiation). Corrosion cracking consists of two main periods: a) crack initiation or incubation period (C), during which primary corrosion-mechanical cracks are initiated on the metal surface under the influence of the localization of the corrosion process and tensile stresses; b) the period of crack development (/p), which, in turn, is determined by the time of subcritical (subcritical) growth of the crack to its critical size, after which avalanche-like destruction occurs. The rate of subcritical growth of corrosion cracks, depending on the material, stress and environment, varies within 10-10 mm/h,
The constant destruction of the film and intense movement of the solution during cyclic loading can dramatically increase the rate of destruction during corrosion fatigue compared to corrosion cracking.
| Rice. 5. The influence of stresses and the type of stress state on corrosion cracking in various environments (solid lines - welded joint, dashed lines - base metal): |
a — aries > 0, <т0СТ — 0; 1,4 - uniaxial bending: 2 - biaxial bending, 3, 5, 6 - uniaxial tension; 1, 2, 3 - 12Х18Н10Т; 4 - OT4; 5 - StZsp; 6 - AMgb; b - (Toast Aries = 0; / - 12X18N10T; 2 - VT1-1; 3 - StZsp; 4 - OT4; c - Ovi > O, (Toast > 0; 1 - 12 XI8N YUT; 2 - OT4; Wednesdays -- see table 3
Fractures are caused by tensile stresses. For most metal-medium combinations, there are threshold values of apore stresses, below which cracking does not occur at all or on a certain test basis (Fig. 5). Threshold voltages vary within the range of (0.2-1) at (Table 2). Welded joints crack more rapidly than the base metal due to the impact of the welding process. Residual welding stress
2. Approximate values of the ratio of threshold stresses to the yield strength of some materials in typical environments that cause their cracking
| DLaterial | Wednesday | Tempera tour, °C | Basic metal | Welded connect nia |
| Carbon and low alloy steels | Alkaline solutions Nitrate solutions Media with hydrogen sulfide | T > 60 °C Boiling Normal | 0.9—1 0.5 0,5—1 | 0,9-1 0,5 0,3—0,5 |
| Corrosion-resistant chromium-nickel steels type 12X18 SHOT: annealed deformed | Chlorides | Boiling | 0,5—0,6 0,2—0,5 | oo 1 1 CHG Xia 0*0 |
| Aluminum alloys | Solutions based on 3% NaCI | Normal Naya | 0,6 | 0.5 |
| Titanium a-alloys | Methanol bromide | 0.5 | OD—0.4 |
Loads without external load can cause intense cracking (Fig. 5, 6). In environments that cause cracking, the effects of residual stresses and stresses from external loads are summed up (Fig. 5, c).
The influence of plastic deformation on corrosion failure is not clear (Fig. 6), since as a result of plastic deformation, both favorable and unfavorable changes in the properties of the metal and the stress state can occur. Unfavorable ones include: a) an increase in the internal energy of the metal; b) the occurrence during deformation of anodic phases, grid defects, micro- and macro-damages of the surface and structure, accompanied
Rice. 6. Influence of preliminary plastic tensile deformation on the time before corrosion cracking of steel StZsp (1), 12Kh18N10T (2), AMgb alloy (5) and compression deformation of AMgb alloy (4). Corrosion tests at constant load o = 0.9
increase and concentration of tension of the second kind; c) the occurrence of intrinsic tensile stresses of the first kind during uneven plastic deformation. Processes that contribute to increasing resistance are:
a) reduction, redistribution and removal of intrinsic stresses of the first kind during the deformation process, as well as the occurrence of compressive stresses on the surface; b) a more uniform distribution of the anodic sections, which reduces the localization of corrosion destruction. Depending on the metal and the environment, the deformation-force scheme, the degree of deformation and thermal deformation conditions, certain processes prevail and the resistance of the metal increases or decreases. The negative influence of concentrators is especially pronounced in environments in which the metal is in a passive or active-passive state. As the aggressiveness of the environment increases, the influence of the concentrator weakens. The higher the level of tension, the stronger the influence of concentrators with relatively less influence of the environment.