Properties of LPG
To understand why propane is mixed with butane, you need to know the characteristics of each component, including their interaction with the external environment. From a molecular structure point of view, they are hydrocarbon compounds that can be stored in a liquid state, which greatly simplifies transportation and operation.
One of the conditions for the formation of liquid gas is high pressure, so it is stored in special tanks under a pressure of 16 bar. The second condition for the transition of hydrocarbon gases from one state to another is the external air temperature. Propane boils at -43°C, while the transformation from liquid to gaseous state in butane occurs at -0.5°C, which is the main difference between these hydrocarbons.
Table with some other properties of these gases
Additional information about the properties of liquefied hydrocarbon gas can be read in the article: propane-butane for a gas holder - properties and application features.
What is butane?
Butane is a so-called liquefied natural (petroleum) gas, which is obtained by distilling oil.
Like propane, butane is a gas in nature, but this is not the case for all flammable gases. Butane becomes liquid at -0.5 degrees Celsius or lower, while propane only becomes liquid at -40 degrees Celsius. The other big difference between butane and propane is pressure: at 20 degrees Celsius, butane has a pressure of about 1.2 bar , while propane is at least 7.0 bar .
Both isomers of butane are gases at room temperature because (n-)butane has a melting point of -138 C and a boiling point of -0.5 C, and methylpropane (isobutane) has a melting point of -160 C and a boiling point of -12 C. Butane is practically insoluble in water (90 mg/l). Both isomers behave in a similar way: they are flammable, do not discolor bromine water and potassium permanganate solution, and are only affected by chlorine and bromine halogens when exposed to light.
Why do they mix propane and butane in an autonomous gas supply system?
Considering the physicochemical characteristics of saturated hydrocarbons, their use largely depends on climatic conditions. Liquefied butane in its pure form will not work at subzero temperatures. Whereas the use of pure propane is contraindicated in hot climates, since high temperatures cause an excessive increase in pressure in the gas tank.
Since it is not practical to produce a separate grade of gas for each region, for the purpose of unification, GOST provides a mixture with a certain content of two components within the established standards. According to GOST 20448-90, the maximum butane content in this mixture should not exceed 60%, while for the northern regions and in the winter season the share of propane should be no less than 75%.
Percentage of gases at different times of the year
By the way, more articles from our blog about gasification are in this section.
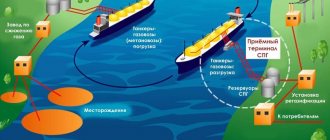
Butane transportation
Butane is transported in liquid form because the gas form is much less compact. 1 liter of liquid butane gives about 250 liters of gas. Therefore, transporting butane in liquid form is much more efficient. The tanker can easily carry 40,000 liters of liquid butane, which corresponds to 10 million liters of butane gas.
The only disadvantage of butane is that it becomes liquid at -0.5 degrees Celsius. Therefore, it cannot be used in cold weather conditions.
Technological factor
In addition to the climate factor, there is a technological justification for why propane and butane are mixed. At oil refineries, during the processing of associated gases, propane and butane are produced in different quantities. Therefore, to optimize the raw material policy, these hydrocarbons are mixed together in a certain proportion. At the same time, regardless of the technology for producing liquefied hydrocarbon gas, the percentage of the two components must be within the limits established by GOST.
Obtaining butane. Chemical reactions - equations for butane production:
Since butane is contained in sufficient quantities in natural gas, associated petroleum gas and is released during the cracking of petroleum products, it is not produced artificially. It is isolated during purification and separation from natural gas, APG and oil during distillation.
Butane in laboratory conditions is obtained as a result of the following chemical reactions:
- 1. hydrogenation of unsaturated hydrocarbons, for example, butene:
CH3-CH2-CH=CH2 + H2 → CH3-CH2-CH2-CH3 (kat = Ni, Pt or Pd, increased to).
- 2. reduction of haloalkanes:
C4H9I + HI → C4H10 + I2 (increased to);
C4H9Br + H2 → C4H10 + HBr.
- 3. interaction of haloalkanes with alkali metal, for example, sodium (Wurtz reaction):
2C2H5Br + 2Na → CH3-CH2-CH2-CH3 + 2NaBr;
2C2H5Cl + 2Na → CH3-CH2-CH2-CH3 + 2NaCl.
The essence of this reaction is that two haloalkane molecules bond into one, reacting with an alkali metal.
- 4. alkaline melting of salts of monobasic organic acids:
C4H9-COOH + NaOH → C4H10 + Na2CO3 (increased to);
C4H9-COONa + NaOH → C4H10 + NaHCO3.
Pricing policy for LPG refueling
The cost of propane-butane depends on the content of the first (more expensive) component. Therefore, it is not surprising that the “winter” mixture for refueling an autonomous gas supply system will be more expensive than the “summer” one. However, if any company offers refueling at a price significantly lower than the market average, then its representative should ask the following questions:
- Why is the cost of LPG so low?
- What is the propane to butane ratio?
- How will this composition work in winter?
- Is appropriate technical documentation available?
- Can I contact the company if problems arise?
Be careful! A cheap mixture can then cost much more.
Some companies cheat by providing a “winter” mixture that does not comply with GOST. Therefore, the low cost of LPG should, at a minimum, alert the buyer.
For autonomous gasification of a home, the quality of gas is of decisive importance, since the reliability of the entire system depends on it. Therefore, it is important to understand why propane is mixed with butane, and in what proportion these components should be used in winter and summer. Read more about gas supply to private properties in the article: autonomous heating with propane-butane.
To avoid problems with gasification of your home, contact a company that has already proven its professionalism and reliability. This is evidenced by our good position in the market and the absence of negative feedback from customers.
Butane, formula, gas, characteristics:
Butane is an organic substance of the alkanes class, consisting of four carbon atoms and ten hydrogen atoms. The name comes from the root “but-” (the French name for butyric acid is acide butyrique) and the suffix “-an” (which means it belongs to alkanes).
The chemical formula of butane is C4H10. It has two isomers n-butane and isobutane. In chemistry, the name "butane" is used primarily to refer to n-butane. The mixture of n-butane and its isomer isobutane has the same name.
Rational formula of n-butane CH3-CH2-CH2-CH3, isobutane CH(CH3)3.
Structure of the n-butane molecule:
Structure of the isobutane molecule:
Butane is a colorless, tasteless gas with a specific characteristic odor.
It is found in nature in natural gas extracted from gas and gas condensate fields, and in associated petroleum gas. To separate natural and associated petroleum gas, they are purified and gas is separated.
It is also formed during cracking of petroleum products, incl. shale oil.
Also found in shale gas and LPG (liquefied natural gas).
Fire and explosion hazard.
Slightly soluble in water and other polar solvents. But it dissolves in some non-polar organic substances (methanol, acetone, benzene, carbon tetrachloride, diethyl ether and others).
It is low toxic, but has a harmful effect on humans - on the nervous system (poisoning, vomiting, death is possible), has narcotic properties, can cause suffocation and cardiac arrhythmia, and causes dysfunction of the pulmonary-respiratory system. Hazard class four.
Chemical and physical properties
Propane-butane is odorless. However, it is highly explosive. Therefore, in order to be able to immediately detect a leak if something happens, a strong-smelling odorant substance, ethyl mercaptan, must be added to this mixture.
Due to the property of the appearance of vapor pressure in the presence of a liquid phase in the cylinder, this gas is convenient to store in small volumes.
Dependence of saturated vapor pressure of propane-butane mixture on temperature
When used as a fuel mixture, butane is quite high in calories, and propane evaporates easily at low temperatures. Therefore, the ratio of these substances in the mixture depending on the temperature conditions plays an important role.
As a rule, the lower the temperature, the more propane should be in the mixture (70-80%). In summer, it is enough for the propane content in the mixture to be about 40%.
Where is propane used?
Due to its high combustion temperature, propane is widely used as a fuel for cars, as a fuel element for gas stoves and heating systems. At enterprises it is used in the process of cutting metal and welding metal structures. In the food and chemical industries it is used as a solvent or food additive.
Propane-butane mixture
Reactions[edit]
Spectrum of a blue flame from a butane burner, showing emission of the CH molecular radical band and the C 2 Swan band
. When oxygen is abundant, butane burns to produce carbon dioxide and water vapor; when oxygen is limited, carbon (soot) or carbon monoxide can also form. Butane is denser than air.
When there is enough oxygen:
2 C 4 H 10 + 13 O 2 → 8 CO 2 + 10 H 2 O
When oxygen is limited:
2 C 4 H 10 + 9 O 2 → 8 CO + 10 H 2 O
By weight, butane contains about 49.5 MJ/(13.8 kWh/kg; 22.5 MJ/lb) or by liquid volume 29.7 megajoules per liter (8.3 kWh/L; 112 MJ/US gal; 107,000 BTU/US gallon).
The maximum adiabatic flame temperature of butane with air is 2,243 K (1,970 °C; 3,578 °F).
n-
Butane is the feedstock for the DuPont catalytic process for producing maleic anhydride:
2 CH 3 CH 2 CH 2 CH 3 + 7 O 2 → 2 C 2 H 2 (CO) 2 O + 8 H 2 O
n-
Butane, like all hydrocarbons, undergoes free radical chlorination, giving both 1-chloro- and 2-chlorobutanes, as well as more highly chlorinated derivatives. The relative rates of chlorination are partly explained by different bond dissociation energies, 425 and 411 kJ/mol for the two types of CH bonds.
How to store propane-butane?
You can learn about the storage features of the substance by following the rules prescribed in GOST. Propane butane must be kept under high pressure in specialized underground storage facilities, tanks or metal cylinders. It is imperative to secure this place so that there are no impacts on the container, overheating and other factors that could cause an explosion. This applies to both full and empty containers. Do not store propane-butane near other explosive substances, toxic and flammable materials, as well as oxidizers.
As you can see, the technical mixture is quite dangerous, but nevertheless very important for the normal functioning of many industries.
Transportation and storage
Since evaporation of liquid in LPG occurs even at 0 °C, these gases are usually stored strictly in closed containers.
Large consumers receive hydrocarbon gases in railway or road tanks, from which they are transferred to factory stationary containers.
Small consumers usually use cylinders.
Transport gases in accordance with the rules for the transportation of dangerous goods and the rules for the safe operation of pressure vessels.
First of all, it is worth considering the properties of propane. Its temperature in the cylinder should not exceed 15 degrees Celsius, otherwise there is a risk of fire.
Isomers [edit]
Main article: C4H10
| Common name | butane normal straight butane n -butane | isobutane and -butane |
| IUPAC name | butane | methylpropane |
| Molecular diagram | ||
| Skeleton diagram |
Rotation around the central C-C bond produces two different conformations ( trans
and
gauche
) for
n
isobutane. [9]