Soldering galvanized iron at home: tips from professionals
Soldering galvanized iron requires a specific approach to the process. Flux is required for this. This substance is both a solvent and an oxidizing agent. Additionally, this substance allows the metal to be wetted with iron, so you can get a high-quality seam. Most often, rosin or hydrochloric acid is used as a flux for working with galvanized parts or products at home. In some cases, it is possible to use boric acid or zinc chloride.
Applying zinc coating at home
- print version
Applying zinc coating at home
Post #1 fox_count » 24 Jan 2021, 01:16
In general, since there are a lot of videos on this topic on the vastness of “these Internets” and there are few specifics, and due to no small need, I decided to try my strengths in this field. I was guided by this popular video and description on the site as a manual for action description https://www.youtube.com/watch?v=dk7uYUpUADw
I took a strip of steel, cleaned it and prayed, first measuring its thickness to get an idea of the rate of growth of the zinc layer. I did everything as in the video and in the description on the site, but it seems there are pitfalls. took several salt batteries and dismembered them
In general, I came to the conclusion that: 1. the battery case is really made of zinc 2. the sulfate must be definitely diluted, but to what extent it is not yet clear (and the bastards do not say anywhere) and it seems that sulfate from sulfuric acid is not the most suitable for this galvanizing operation. only costs. I'll try it in the soldering room tomorrow. 3. It’s not clear why, but the rag greatly interferes with the process even though it’s made of cotton.
PS: Dear fellow experts, can anyone tell me what I’m doing wrong besides the thickness of the selected wires and sulphate from sulfuric acid, as well.
Applying zinc coating at home
Post #2 AnSm » 24 Jan 2021, 01:42
Applying zinc coating at home
Post #3 fox_count » 24 Jan 2021, 01:59
Applying zinc coating at home
Post #4 AnSm » 24 Jan 2021, 02:01
I don’t know why it says this, but the video uses zinc chloride, not sulfate. I'm not a chemist, but the electrolyte must be precise for electroplating, otherwise nothing will work. I'll look into galvanizing now, maybe I'll find something.
Sent after 1 minute 39 seconds:
Applying zinc coating at home
Post #5 fox_count » 24 Jan 2021, 02:10
Applying zinc coating at home
Post #6 AnSm » 24 Jan 2021, 02:15
Here's a little about galvanizing in general and home galvanizing in particular. This article tells you how to galvanize with zinc sulfate, but the composition is slightly different and consists of three components. That is, it is more difficult than zinc chloride. Video of how easy this is to do with zinc chloride (soldering acid). https://www.youtube.com/watch?v=SeV6pao9yG8 And here we talk in more detail about home galvanizing. And again with soldering acid. https://www.youtube.com/watch?v=1E7dTMRcB34 Here are the above videos and a description in more detail about the types of galvanizing.
When is galvanized metal suitable for soldering?
To correctly solve the question of how to solder galvanized steel, it is necessary to consider some of the properties of zinc. This metal begins to melt at a temperature of +460 o C. And at a temperature of +960 o C it begins to evaporate. Above these temperature values, pores, cracks and solder joint defects begin to form in the material. Therefore, the procedure can be carried out only with lower rates. An alternative is to use filler wire. In industrial conditions, the procedure in this case is carried out in a protective gas environment. Wire containing copper with silicon, bronze and aluminum is more often used.
These materials provide the following advantages:
- the welding seam is protected from corrosion;
- Spattering during soldering is minimal;
- the coating fades slightly;
- the procedure requires low heat levels;
- processing the formed seam is simple;
- Natural cathodic protection is formed in the weld area.
Solder for household work, its composition and properties
Solders are usually classified into hard and soft. For soldering galvanized steel at home, only the second group is used. If you use hard solders, it is not only impossible to achieve a high-quality weld, but there is also a risk of warping of the galvanized iron products themselves. Filler materials must have a low melting point, the point should be lower than that of the base material. Most often, POS-30 solder is used at home; it is a tin-based substance. It is better to use zinc chloride as a flux. If the surfaces have been tinned in advance, then rosin can be used. POS 30 is characterized by the following properties:
- optimal fluidity, materials penetrate into all spaces, filling even small voids;
- relatively low melting point;
- POS 30 are produced in various standard sizes, which allows you to select the optimal modification for specific work;
- a high degree of wettability facilitates the process and guarantees higher quality results;
- materials can be used for tinning workpieces;
- POS 30 has good conductivity and low resistance, which allows it to be used for soldering small parts;
- After hardening, the materials rigidly fix the parts together.
The connections are smooth and tight. The seams are balls on top of the base material.
If the elements to be soldered are large, then before soldering they need to be tinned - cover the surfaces with a thin layer of solder. The same action is necessary when soldering cylindrical products that fit into each other. If these are pipes, then solder is applied to an element with a larger diameter from the inside, and for a part with a smaller diameter - from the outside.
POS 30 consists of 30% tin and 70% lead. The material has the following technical parameters:
- the material begins to melt at +180 o C;
- complete melting of POS 30 occurs at a temperature of +256 o C;
- density – 10.1 kg/m3;
- alloy elongation in relative terms – 58%;
- crystallization interval – 73 o C;
- tensile strength – 32 mPa.
Soldering galvanized iron at home: tips from an expert
Soldering galvanized iron requires a specific approach to the process. Flux is required for this. This substance is both a solvent and an oxidizing agent.
Additionally, this substance allows the metal to be wetted with iron, so you can get a high-quality seam. Most often, rosin or hydrochloric acid is used as a flux for working with galvanized parts or products at home.
In some cases, it is possible to use boric acid or zinc chloride.
When is galvanized metal suitable for soldering?
To correctly solve the question of how to solder galvanized steel, it is necessary to consider some of the properties of zinc. This metal begins to melt at a temperature of +460 oC. And at a temperature of +960 oC it begins to evaporate.
Above these temperature values, pores, cracks and solder joint defects begin to form in the material. Therefore, the procedure can be carried out only with lower rates. An alternative is to use filler wire.
In industrial conditions, the procedure in this case is carried out in a protective gas environment. Wire containing copper with silicon, bronze and aluminum is more often used.
These materials provide the following advantages:
- the welding seam is protected from corrosion;
- Spattering during soldering is minimal;
- the coating fades slightly;
- the procedure requires low heat levels;
- processing the formed seam is simple;
- Natural cathodic protection is formed in the weld area.
Solder for household work, its composition and properties
Solders are usually classified into hard and soft. For soldering galvanized steel at home, only the second group is used. If you use hard solders, it is not only impossible to achieve a high-quality weld, but there is also a risk of warping of the galvanized iron products themselves.
Filler materials must have a low melting point, the point should be lower than that of the base material. Most often, POS-30 solder is used at home; it is a tin-based substance. It is better to use zinc chloride as a flux. If the surfaces have been tinned in advance, then rosin can be used.
POS 30 is characterized by the following properties:
- optimal fluidity, materials penetrate into all spaces, filling even small voids;
- relatively low melting point;
- POS 30 are produced in various standard sizes, which allows you to select the optimal modification for specific work;
- a high degree of wettability facilitates the process and guarantees higher quality results;
- materials can be used for tinning workpieces;
- POS 30 has good conductivity and low resistance, which allows it to be used for soldering small parts;
- After hardening, the materials rigidly fix the parts together.
The connections are smooth and tight. The seams are balls on top of the base material.
If the elements to be soldered are large, then before soldering they need to be tinned - cover the surfaces with a thin layer of solder. The same action is necessary when soldering cylindrical products that fit into each other. If these are pipes, then solder is applied to an element with a larger diameter from the inside, and for a part with a smaller diameter - from the outside.
Equipment for work at home
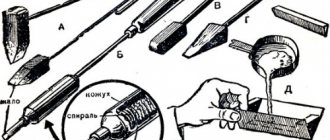
Before wondering how to solder galvanized iron at home, you need to prepare the necessary equipment. The main tool is a regular soldering iron with an awl-shaped tip. But other devices will also be useful. A soldering iron requires a special holder or stand that will hold the tool in a heated state. To accurately connect small parts, you will need tripods with optical lenses. To remove smoke from the room - smoke absorbers. Tin pumps will be needed to remove excess tin. There are various switches, thermal pastes, control modules and adapters. This equipment will not only allow you to perform the soldering process, but will also provide the highest quality results.
Soldering at home
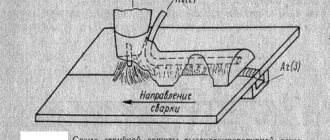
One of the oldest and fairly simple methods of reliably connecting metal parts is the so-called soldering, used in the manufacture of any product using surface diffusion, as well as when melting an intermediary metal.
This method is widely used, as a rule, when it is necessary to repair technical devices and household items. Electrical and radio engineering is the most common area of application of soldering. The positive aspects of soldering are their simplicity and universal availability, ease of repair, dismantling, and water resistance.
Soldering iron with tin solder
The soldering process is the chemical joining of two metals using solder. Moreover, the crystal structure of the metal does not change. That is, the connected parts remain with their technical characteristics.
The connection itself is quite reliable, but much will depend on the type of solder and soldering technology. In addition, it should be noted that not all metals can be joined by this process. Basic metals, especially steel (iron), can be soldered together.
Three technologies
There are three technologies for soldering iron with tin:
- soldering iron To do this, you will have to use soft solders with a high lead content;
- blowtorch. This will require hard solders with a high tin content;
- electrical soldering of iron.
The first method is used if the iron will not be subjected to heavy loads during operation. The second is tinning of iron with tin, when tin solder is applied to the surface of a metal product and rubbed over its entire plane in a thin layer.
This technology requires soldering flux. The third option is used on a production scale, for which special equipment is used.
Soldering sheet metal
Soldering tin (thin sheet iron) is a common process in the manufacture of metal containers. But often, even at home, it is necessary to fasten sheets of iron together, assembling sealed structures. Therefore, before soldering one sheet to another, you need to prepare everything you need.
For the process of soldering iron with tin, you will need solder with a small concentration of tin, for example, POS-40, flux, a soldering iron and an awl.
In the process of soldering iron, flux acts as a solvent and an oxidizing agent at the same time. That is, the metal is immediately wetted and protected from oxidative processes. Rosin and hydrochloric acid or zinc chloride and boric acid are used as fluxes.
As for the soldering iron, for high-quality tin soldering it is better to choose an electric tool with a power of more than 40 W. The old soldering tool, which is heated by the flame of a fire, is practically not used today, even at home.
About soldering of tin products
Solder POS-40 with a rosin channel.
This process is quite common in the manufacture of metal containers. However, often in everyday life you may encounter the problem of joining sheets of tin. An important point of the task is the need to form a reliable sealed seam.
When soldering products made from cold-rolled sheet iron using tin solder, you should have an alloy with a low stanum content, for example POS-40 or POS-18, flux, a soldering iron, and an awl.
Flux is used as a solvent and oxidizing agent. Thus, in this case, wetting and protecting the product are carried out simultaneously. Rosin, hydrochloric acid or zinc chloride are perfect for this type of material.
The most suitable tool for carrying out the soldering process is a regular soldering iron with a power of at least 40 watts.
Sequencing
Here are the main stages of this process:
- cleaning of joined sheets;
- applying flux;
- heating up the soldering iron and tinning;
- soldering with tin;
- cleaning the joint with gasoline.
Cleaning is carried out mechanically with sandpaper. If the contamination is large, you will have to treat it with a solvent. If it is not possible to clean it using this method, then etching is carried out with sulfuric acid.
Two pieces of sheet iron are brought to each other at a distance of 0.3 mm. Their edges are treated with paste-like flux using a brush. The soldering iron tip is cleaned with sandpaper, and the tool itself is plugged into the electrical network through an outlet. To check whether it has heated up well, you need to place its tip in the ammonia mixture, which should boil.
Now the stage of tinning the iron is carried out. That is, using solder made of tin or its alloy, the edges of two sheets of tin are processed in order to cover them with a tin layer, which will perform protective functions against metal corrosion.
Everything is ready, all that remains is to solder the two ends of the sheets. The soldering iron tip is brought to the joint along with the tin solder, and they both move smoothly along the joint boundary.
Process technology
The tin parts are connected in accordance with the following steps:
- cleaning of parts;
- applying flux;
- soldering iron preparation and tinning;
- soldering;
- processing the formed compound with gasoline.
Properties of various brands of solders.
The joints can be cleaned mechanically. For these purposes, you should use sandpaper or a wire brush. If there are serious contaminants that cannot be removed by simple methods, you can use solvents.
If this method turns out to be ineffective, then you should resort to hydrochloric acid.
Then they proceed directly to the connection. The iron sheets are brought closer to each other by three tenths of a millimeter. The edges of the parts must be treated with flux. It is important to remember to clean the soldering iron tip before use. This cleaning can be done using simple sandpaper.
Now the tinning stage is carried out. Using the above-mentioned POS, the edges of the product are processed. This is necessary to cover their surface with a layer of tin, which performs protective anti-corrosion functions.
All preparatory procedures are ready and you can solder the two parts together. The soldering iron tip is brought directly to the joint and a joint is formed using tin solder.
It is important to understand that soldering iron with tin is a process that requires compliance with safety precautions. In this regard, basic precautions should be followed. Hands must be protected with special gloves.
Don’t forget about the stand for the soldering iron to eliminate the possibility of damage to the working surface as a result of contact of a heated tip with a table or improvised tool.
At first glance, this procedure may seem quite simple. However, everything is not so simple. It should be treated with due care. After all, even minor errors in soldering can lead to the formation of a low-quality seam that does not meet the expected requirements.
Features of working with galvanized products
Soldering galvanized tin in a purely technological process is no different from the previous one. But the technology has its own subtle nuances that affect the quality of the final result.
You cannot solder galvanized steel with solders that contain large amounts of antimony. This substance, when in contact with zinc coating, creates a weak seam.
It is better to use boric acid and zinc chloride as flux. If the products themselves have already been tinned with tin during the production process, then rosin can be used as a flux.
When a connection is made between galvanized iron (sheet) and wire, the latter must be bent at a right angle to increase the contact area of the two products.
The rest of the process is carried out exactly the same. By the way, it doesn’t matter whether the wire was made of galvanized or ordinary steel.
There are several more important points that must be taken into account in the process of soldering galvanized products. If solder rods based on tin and lead are used for soldering iron, then it is better to add flux based on zinc chloride and ammonium chloride. The ratio is 5:1 respectively.
Tin and cadmium based solder requires caustic soda as a flux additive.
If galvanized iron products, the protective layer of which contains more than 2% aluminum, are connected to each other, then solder based on tin and zinc is used. And hydrochloric acid and petroleum jelly (stearin) are used as flux.
Regardless of which parts or assemblies are connected by soldering, it is necessary, after the process is completed and the seam has cooled, to rinse the joint with water to remove any remaining flux.
Soldering sheet metal at home - Machine tools, welding, metalworking
Both in production and at home, people are faced with the need to solder parts made of tin, which has its own characteristics and preparation.
In fact, tin is cold-rolled sheet steel, which is subsequently subjected to heat treatment and galvanic coating in the form of tin, zinc, chromium and other materials.
Tin (white and tinned) is used in the production of cans for cold drinks or metal containers of any size. In this way, lightening of the packaging body is achieved. Therefore, correct soldering of this metal is important.
Materials for soldering metal
The classic version of soldering sheet metal requires solder containing tin and other chemical elements, flux, and a soldering iron with an awl.
According to the recommendations, solder of the POS 40, POS 30 and POSS 4-6 brands should be used. This is due to the chemical properties of the materials during the tin soldering process. Each solder may contain several elements, including tin, antimony, arsenic, copper and bismuth.
These solders differ from others in shear resistance due to the impurity content. For example, POS 40 contains 40% tin, 2% antimony, 0.05 - 0.1% of the remaining elements. It also increases the tensile strength of the seam after soldering. When tin decreases in the composition, antimony is increased.
But it also happens that soldering requires the presence of a component such as lead (POS 90). In the case of galvanized material, the situation is different.
Soldering iron requires the presence of flux. Essentially, it is a solvent and a chemical oxidizing agent. During soldering, thanks to this element, oxidation does not occur.
It also ensures that the metal is wetted with iron for better weld quality. Popular fluxes for metal are hydrochloric acid and rosin. The latter is actively used in the radio engineering industry.
Zinc chloride and boric acid are sometimes used.
It will not be possible to select the most suitable flux for soldering parts made of tin, since each of them gives a positive result. If it is necessary to dissolve thick fatty substances, ammonium chloride is used. Often, mixtures of the above components are made for this procedure.
An important role in the process of soldering with tin is played by the tool used, which is usually a soldering iron.
According to recommendations, its power should be more than 40 W. It is advisable to use an electric soldering iron. In this case, soldering will be convenient, and the resulting seams will be strong and reliable. It should be noted right away that you should not leave the tool in a heated state unnecessarily in order to avoid a fire, as well as deterioration of the properties of the handle.
Soldering stations are used in industry, the cost of which is several times higher than classical equipment, but the products have various attachments, as well as additional elements (stand, temperature sensor, etc.).
Soldering process of metal parts
Soldering involves several stages, during which a high-quality seam is ensured. To solder metal you need:
- surface cleaning;
- degreasing;
- applying flux;
- preparing a soldering iron;
- tinning the place of the future seam;
- soldering of tin plate elements;
- cleaning the surface with a gasoline mixture;
- control of the resulting seam.
Cleaning of surfaces is ensured by the following tools:
The gap between the parts must be 0.3 mm for capillary forces to occur. This situation allows the metal to fill the edges of the gap and provide a quality seam.
Sometimes parts cannot be cleaned mechanically and etching is used, but in the case of tinplate this is a rare option. If there are grease stains on the surface, it is necessary to use a soda solution (10%).
At home, degreasing is done using acetone, gasoline or alcohol-gasoline mixture. Their properties ensure excellent cleaning.
The next stage of soldering tin metal is the application of flux. As a rule, this is done with a brush or rag. The flux is stored in ordinary containers at room temperature. Soldering involves abundant wetting of the seam area with this component.
In the process of preparing the soldering iron, it is necessary to ensure that the surface is clean so that in the future the solder can spread freely along the working plane. To do this, use coarse sandpaper or a regular file to sharpen the tip of the tool.
Then it is plugged into the network and warms up. Periodically, the tip is moistened with ammonia tincture to avoid unnecessary contamination. By the way, it is with this mixture that the heating level of the instrument is checked.
When the soldering iron is well heated, the ammonia on the surface begins to hiss and becomes covered with a greenish-blue tint.
Next, tinning is carried out. It should be noted that when soldering tin parts such as cans, this step is skipped, since it is included in the manufacturing technology.
It provides increased density and strength of the seam. An important aspect is that the process is performed with the same material that is used during soldering.
Thus, if metal soldering is carried out by POS 30, then tinning is also carried out with this solder.
The process of soldering metal products itself involves securing the elements for the convenience of creating a seam. One piece of tin is placed on top of another, or a small gap is formed between them.
You can hold the edges using an awl or other similar tool.
The tip with solder is brought to the iron products and passed along the intended seam with smooth movements. Consolidation plays an important role in this case, since in the event of a potential shift, the process will have to be repeated again.
Thus, the required amount of solder is applied to the surface, forming a high-quality seam. The soldering iron should be pressed with the entire edge, and not with its individual end.
During this process, the elements of the sheet metal are also heated, due to which the seam is well attached to the surface.
After soldering is completed, the resulting metal layer should be treated with a gasoline mixture or alcohol to reduce the heat concentration and clean it from solder and acid residues. This helps prevent rust from occurring in the future.
Surface inspection is performed visually. Microscopes and magnifying glasses are used on an industrial scale. The seam should be glossy, without pores or cracks, covering only the required surface. Only in this case is the soldering considered to be of high quality.
Features of galvanized parts
For soldering galvanized sheets, POS 30 and POS 40 solders should be used. This is due to the fact that POSS 4-6 contains a large amount of antimony, which deprives the subsequently formed seam of strength and elasticity.
When soldering galvanized parts, a solution of zinc chloride is used according to the recommendations. In the case where the surface was tinned in advance, you can use rosin flux and not wash the product after soldering.
Soldering is otherwise ensured by the same technological process as described above.
Actions with wire
If you plan to solder wire to sheet metal, both galvanized and regular, you should first bend one of the corners at an angle of 90 degrees. This will ensure the strength and reliability of the structure. The process is completely similar to the previous description.
Precautionary measures
Be sure to use personal protective equipment. For the soldering iron, use special stands so that the tip does not touch the objects at hand. This may result in damage or an emergency.
Safety precautions
Soldering iron with tin is an unsafe process. Therefore, precautions must be strictly observed. Protective gloves are put on your hands, and a stand must be installed under the soldering iron so that the heated tip does not touch the table and available materials. And the procedure itself must be carried out carefully.
Despite the apparent simplicity of the soldering operation, it is actually a serious procedure. And you need to treat it with great attention. Something was missed, they were even applied incorrectly, and we can assume that the quality of the joint has dropped sharply. Therefore, it is important to approach each stage responsibly, especially when it comes to cleaning two joined iron products.
How to seal a galvanized seam hermetically?
Hello. Actually, subzh: How to seal a galvanized seam hermetically?
There is a galvanized pipe with a diameter of 100 mm, galvanized. As usual, bent from a sheet, the seam is straight, I don’t know what roofers call it, but edge by edge and tapped with a hammer. An example is a seam on a bucket. Fresh galvanized.
When pouring water inside (without pressure), water leaks out, where it is not clear, but from below the seam it drips very intensively (it drips, it does not flow).
Question: is it possible to seal the seam hermetically (so that water does not drip while pouring)? At the same time, a question arose: do the seams on the buckets not leak? Nobody solders them. How are they made?
It is advisable to solder with a gas torch, because the length is in meters, fiddling around with a soldering iron takes a long time and the result is unclear. If there’s no way to use a burner, then maybe. The pipe has not yet been installed, i.e. can be rotated in different directions. Naturally, you can’t crawl inside, i.e. The seam must be soldered from the outside. How to achieve capillary flow of solder into the seam without discontinuities?
I tried it this way: I heated it with a torch and poked it with a solder wire along the seam. it seems to be soldered, the solder melts, the seam seems to close - everything is just like for real, but when you pour water it still drips.
What's the trick? How to seal so that water does not drip? There will be no load, no pressure either.
Who did the soldering themselves? I ask for advice only from truly experienced gentlemen.
You can solder with a torch, I soldered with a solid tertiary (with lead). but the pipe must be preheated, and for a very long time. on buckets, a sealed seam is obtained by competently “flexing into a lock” » > .
sanya1965 wrote: you can solder with a torch, I soldered with a solid tertiary (with lead). but the pipe must be preheated, and for a very long time. on buckets, a sealed seam is obtained by competently “flexing into a lock” » > .
Well, of course. The pipe was bought ready-made, but I didn’t make it myself - I laid a sheet around the log and that’s it. l with a hammer a couple of times. This is exactly the castle that is present. Looks like the real thing. However, what does “need to be heated for a very long time” mean? What is it that is necessary and very necessary? Where is the criterion for sufficient warming up? I heated it up, and everything seemed to be soldered very well, but it was not hermetically sealed.
You say, you soldered, and remind you that you can solder with a torch. So the Internet assures us that soldering is easily no problem. Did anyone doubt that it was possible? What did you solder with? Soldering iron?
When heated with a burner (blue reducing flame), the color of the surface does not visually change, so it is not clear how to determine the degree of heating. In addition, I noticed that when heated, the zinc from the coating melts and runs down in tears. My solder spreads perfectly over the surface and flows into the seam. Everything is grown-up. Only the water is dripping. Doesn't know about soldering.
At 450 Celsius, zinc melts, at 800 degrees it already evaporates. Both temperatures are nonsense for the burner and cannot be visually distinguished.
I am asking for practical advice on how to achieve the formation of a sealed seam when soldering a galvanized roof lock with a gas BURNER. From those who really know how to solder. In Moscow I am ready to pay money for hermetic soldering or for science. Put on the beer.
T-34 wrote: How to achieve capillary flow of solder into the seam without discontinuities?
Use flux for soldering
T-34 wrote: You say you soldered. How? Soldering iron?
Two 100-watt. What kind of flux do you have?
Victorych wrote: Two 100-watt ones. What kind of flux do you have?
I have FIM. According to the description, phosphoric acid (apparently orthophosphoric acid).
I realized that you soldered with a soldering iron. But I say again - I need to solder with a gas BURNER.
The acid evaporates almost instantly, leaving black boogers on the surface.
T-34 wrote: phosphoric acid
Buy flux for soldering copper pipes at a plumbing supply store. the thing is wonderful in all respects - a paste with fine solder crumbs, with acid, so it’s better to rinse it with a soda solution. Although, I’ve seen plumbers, they don’t wash anything. Buy it there and solder it. Almost everything is soldered with this flux, not luminium, of course, but for example, I soldered nichrome with it. And in general, I always have this flux on hand. Well, also go over the seam with a small cord brush first. You can solder with a gas torch or an electric soldering iron. I sometimes use an old iron to heat it next to or below the seam.
T-34 wrote: But I say again - I need to solder with a gas BURNER.
Buckets were soldered with a copper “hatchet” heated by a blowtorch. You can heat with a gas burner. You can use rosin, borax, or neutral solder fat as a flux. More details here: » >
volodrez wrote: Buy flux for soldering copper pipes at an engineering plumbing store. the thing is wonderful in all respects - a paste with fine solder crumbs, with acid, so it’s better to rinse it with a soda solution. Although, I’ve seen plumbers, they don’t wash anything. Buy it there and solder it. Almost everything is soldered with this flux, not luminium, of course, but for example, I soldered nichrome with it. And in general, I always have this flux on hand. Well, also go over the seam with a small cord brush first. You can solder with a gas torch or an electric soldering iron. I sometimes use an old iron to heat it next to or below the seam.
Guys, who soldered galvanized steel with a torch? What does flux for copper have to do with it? I am very good at soldering copper, including with a torch. Who soldered a galvanized seam themselves? The issue of servicing does not raise any questions. The issue is the tightness of the soldering so that water does not drip. Heat it with an old iron - are you even reading the question, what is it about? Or is it not important to you? Pipe with a diameter of 100 mm.
Soldering galvanized steel at home
Guest
Group: Participant Messages: 84 User No.: 30371 Registration: 13-March 08
you need to solder the seam on the zinc-coated roofing iron body.
The POS61 solder does not want to stick. Fresh galvanized. I cleaned the soldering area.
Used: 1. Technical hydrochloric acid 2. -//- + zinc 3. Phosphoric acid 4. Flux F-38N 5. Flux F-61A
Someone actually soldered. After all, the coffins are soldered.
Partner in crime
Group: Participant Messages: 1111 User No.: 27717 Registration: 26-December 07 Place of residence: Kaliningrad region
But doesn’t soldering acid solder (Zn)? In the description it solders almost everything from cast iron to brass, but zinc is not written, but before it looked like galvanized buckets were soldered
This post has been edited by xzxzx
— Dec 14 2009, 07:49 PM
Grandfather
Group: Author Messages: 20684 User No.: 27360 Registration: 16-December 07 Place of residence: Ukraine
“Perfection is achieved not when there is nothing to add, but when there is nothing to take away” /Antoine de Saint-Exupéry/
How to take a photo of your device with your phone?
https://vrtp.ru/index.php?act=categories&CO.
le&article=2104 I will develop a device on a microcontroller for you
Administrator
Group: Admin Messages: 5065 User No.: 1 Registration: 19-July 04 Place of residence: Voronezh
Grandfather
Group: Author Messages: 6126 User No.: 38411 Registration: 26-October 08 Place of residence: Ukraine
Grandfather
Group: Admin Messages: 13631 User No.: 20356 Registration: 26-April 07 Residence: Tankograd
Guest
Group: Participant Messages: 84 User No.: 30371 Registration: 13-March 08
The situation is exactly the same. Only the galvanization is completely fresh, the temperature is normal, the same thing rolls off. F61A flux solders aluminum easily, but here it is galvanized. As I understand it, the most “vigorous” one is f64, I’ll go in and buy it.
This post has been edited by KES
— Dec 15 2009, 07:11 AM
Grandfather
Group: Admin Messages: 13631 User No.: 20356 Registration: 26-April 07 Residence: Tankograd
Then I came to my senses, and after a day of rustling around Yandex, I found in one place a mention of ammonia, also known as an aqueous solution of ammonia. Using the trial and error method, I began to do the following: 1. Heated the area with a hairdryer at a temperature of 200-300 degrees (The solder is more refractory than POS61, I needed something stronger and cheaper) 2. I moistened the cotton wool with a 10% ammonia solution and wiped the soldering area. It was clear that the surface was changing (you can wipe it before heating if it makes it brighter). 3. took a 100 W soldering iron and placed it next to the soldering area 4. moistened the cotton wool with soldering acid and wiped the soldering area, or dripped it and waited until the acid almost boiled away. the surface became gray. 5. I used a soldering iron with lightning speed and the galvanization was tinned.
The result can be seen in the photo, I don’t know how long it will last. I spent 20 grams of solder on this place.
if you moisten it with acid and apply a soldering iron, the acid boils away without having time to react. I periodically needed to tin the soldering iron in ammonia, because... the tip becomes covered with soot and the solder does not stick and this makes it worse for heating. During such work, you can inhale toxic fumes to an altered state of consciousness.
They wrote on the Internet that you need to mix soldering acid and ammonia, I haven’t tried it because... I couldn't find the proportions. I considered the existing solutions to be too diluted with water, which greatly cools the soldering area during use.
I would be grateful if knowledgeable people could suggest anything else worthwhile.
Attached image (Click to enlarge)
Uncle Fyodor, you are eating your sandwich on the wrong side. Unforeseen expenses again ————————————————————————- The cat
four legs: input, output, ground and
power
How to solder galvanized steel at home? — Handyman's Handbook
> Electrician's tips > How to properly solder with a soldering iron with acid
Most often, for soldering printed circuit boards in radio engineering products and household appliances, they prefer to use ordinary pine resin rosin, but it can be replaced with other components. When molten, it promotes the spreading of tin solder along the copper traces of the board.
This allows you to reliably solder the legs of radio components and the ends of connecting wires. Rosin allows you to effectively solder copper, tin and silver products.
In order to solder galvanized and stainless iron, radiators, buckets, pans, various alloys, brass and other metals, you can use acid solutions.
Bottle with acid solution for soldering metals
Acidic solutions
It is important to choose the right acid solution. It depends on the type of metal from which the parts are made. This could be an aluminum or copper radiator, a kettle that needs to be soldered, copper, brass or roofing iron:
- Galvanized iron. Places where it is necessary to solder are treated with an acid solution, correctly called (zinc chlorate). This composition can be bought in specialized stores; the easiest way is to prepare it yourself.
To do this, it is enough to throw pieces of zinc into 100 ml of hydrochloric acid, which can be removed from the body of AA batteries. After the chemical reaction is completed, the zinc will dissolve, releasing a large amount of hydrogen.
It is correct to carry out the process in a well-ventilated area, in the absence of open flame.
After the solution has cooled and settled, the upper transparent yellow part is poured into a clean glass container. The sediment is poured into the ground; it is not recommended to drain into sewers with metal pipes. Acid can damage pipes and seals. The remaining part of the solution is ready for processing galvanized iron roofing.
How to solder sheets of roofing iron
- Stainless steel. Before soldering, the surface is cleaned and treated with phosphoric acid, which contains the following elements:
- up to 50% zinc chloride;
- ammonia up to 0.5%;
- dissolves in water with a pH concentration of 2.9%.
Phosphoric acid is used for soldering as a flux and for cleaning metal from rust.
The solution can be transparent, light yellow or colorless; when heated to 213ºC, it is converted into H4P2O7 (pyrophosphoric acid), which degreases the surface of metals. The composition dissolves the oxide film on various metals and alloys:
- stainless steel;
- brass;
- Nickel alloys;
- copper alloys;
- alloys of carbon metals and low-alloy steel.
Application of acids
To solder metal products (pipes, radiators, buckets, pans), the surface of the elements is thoroughly cleaned, using a file or sandpaper. An acid solution is applied to the cleaned areas with a brush, after which solder is melted to a liquid state on the surface with a soldering iron.
How to solder correctly with a soldering iron
Liquid solder tins the cleaned areas; when boiling, the acid flux comes to the surface. When the solder hardens, the soldered elements are securely and hermetically fixed.
You can solder with a powerful soldering iron or an open flame from a gas burner. Various heat sources can be used depending on the surface area to be heated and the melting point of the solder.
Remains of acid flux are washed off with water, preferably a soapy, alkaline solution, this will prevent further corrosion of the metal.
Machined and brazed stainless steel elements
Acid can damage skin and muscle tissue, and inhaling vapors can damage the respiratory organs. When contacting air, hydrochloric acid enters into a chemical reaction, and smoke is visible above the open container. To work correctly in these conditions, wear safety glasses, rubber gloves, a gas mask, or a respirator.
If the solution gets on the skin, wash this area of the body with a 6% alkaline solution or plain soap. It is not recommended to solder radio circuit boards with fluxes containing acid. The acidic components are difficult to wash off and contribute to the breakdown of copper tracks. It is better to replace them; there is a special paste for this.
Soldering acid solutions should be stored correctly in containers made of the following materials:
- glass;
- ceramics;
- porcelain;
- fluoroplastic
Such dishes do not react with acid; the prepared composition can be stored in it for a long time.
Soldering without a soldering iron
At home, if you don’t have a soldering iron, you can solder copper wires with a diameter of up to 2 mm. For soldering radiators and utensils, special solder, blowtorches, and gas torches are used, since the copper rod of the soldering iron is not able to heat a large surface area. There are several ways:
- Tinning and soldering wires in molten solder . The wire is first heated, applied to a piece of rosin, it melts and spreads evenly over the surface of the connection. The wire is twisted and lowered into molten solder in a tin can over a fire; it can be heated with a blowtorch. In order to solder the twist, it is advisable to hold it in boiling tin for up to 1 minute. The copper wires will heat up and the alloy will fill all the gaps between the twisted wires. In this way you can solder small parts made of copper, brass and other alloys.
Tinned and soldered copper wire
- Soldering wires in the trench . The stripped and twisted wires are laid in a 2-3 cm piece of aluminum tube, 0.5-1 cm in diameter, sawn lengthwise. The top is filled with a mixture of fine shavings of solder and rosin dust, and from the bottom this structure is heated with a lighter, candle or small blowtorch.
Heating the solder with a blowtorch (torch)
The mixture melts and thoroughly envelops all wire connections. After hardening, the aluminum gutter is removed and the joint is insulated.
Solder shavings can be sharpened with a coarse file.
- A thin copper wire up to 0.75 mm can be laid on aluminum foil, sprinkled with a mixture of rosin and tin shavings, wrapped tightly and heated for 3-4 minutes. The solder will evenly fill all the elements at the soldering site; after cooling, the foil can be removed and discarded.
How to prepare solder paste
Soldering paste is sold in radio parts stores, but you can prepare it yourself. To 32 ml of hydrochloric acid add 12 ml of ordinary water, then pieces of zinc - 8.1 g. For this, enamel dishes are used.
How to tin a soldering iron: preparation and care of the soldering iron
After the dissolution reaction is completed, tin – 8.7 g is added to the composition. When the second dissolution reaction is completed, the water is evaporated to a paste-like consistency of the solution. The paste is transferred to a porcelain container, where powder is poured, which contains:
- lead – 7.4 g;
- tin – 14.8 g;
- dry ammonia – 7.5 g;
- zinc – 29.6 g;
- rosin – 9.4 g.
This paste is mixed with 10 ml of glycerin, heated and stirred.
How to solder correctly, sequence of actions:
- The parts at the soldering site are cleaned, the wires are twisted;
- the paste is applied with a brush in a thin layer;
- the surface for soldering is heated with a plasma lighter, torch, candle or alcohol tablet, or even with matches or over a fire until the paste melts;
- After melting, the soldering elements are removed from the heat source, and the solder hardens.
Soldering galvanized iron - Soldering
Soldering galvanized iron
When assembling products made from carbon steels coated with zinc using the hot-dip method (zinc plating), soldering is often used. To obtain a shiny surface, 1% tin, lead or aluminum is added to the bath. Sheets coated with zinc using the galvanic method are also produced. Galvanized iron in the form of sheets 0.25-3.5 mm thick has a coating weight of 400 to 850 g per 1 m2.
Soldering galvanized iron sometimes causes difficulties due to the fact that the zinc coating is difficult to wet with tin-lead solder. Overheating the soldering iron during soldering in order to improve wettability only increases the difficulty of soldering.
To select a flux on galvanized iron samples, we tested: 1) concentrated hydrochloric acid; 2) a concentrated aqueous solution of zinc ammonium chloride and 3) acidified flux based on zinc and ammonium chlorides with the addition of tin chloride.
Tin-lead solders containing 60, 40 and 20% tin and an alloy containing 82% cadmium and 18% zinc were tested for spreadability and strength properties of the solder joint. It has been established that solders with a composition of 60% tin and 40% lead or 50% tin and 50% lead, in combination with acidified flux based on zinc chloride, ammonium chloride and tin chloride, are easy to use, spread well and give the strongest connections.
The following soldering process is recommended. Flux is applied to the surface and a short period of time is allowed for the reaction to occur. Heating and introduction of solder are carried out with a large soldering iron, lightly rubbing the joint surfaces. Before starting the soldering process, heating with an open flame is not recommended, although in some cases a gas burner can be used. Remaining flux should be completely removed.
Solder for soldering galvanized iron
O DESCRIPTION 40768
INVENTIONS
TO THE AUTHOR'S SVI IVLSTS
Union of Soviets
Socialist
Republics
Automatic dependent certificate no.
Announced 10.IV. 1972 (No. 1770736/25-27) with the addition of application No. —
Priority
Published 10.X11 1973. Bulletin No. 47
Date of publication of the description: 5X.1974.
M. Kl. At 23k 35/26
From 22 from 11/00
State Quintet
Sovvta Mnnkotrov SSS9 oo donam inventions l discoveries
UDC 621.791.3(088.8) Authors of the invention
V. M. Polyakova, I. E. Petrunin, P. P. Ponamarev and A. A. Ryabtsev
All-Union Correspondence Mechanical Engineering Institute
Applicant
Subject of the invention
The invention relates to the field of soldering.
Solder is known with the following composition, %: cadmium 10 - 20, zinc 0.5 - 15, lead - the rest.
To improve the quality of the soldered joint, the following % were introduced into its composition: nickel 0.1 - 0.3 and copper 0.1 - 0.2, and the remaining components were taken in the following ratio: cadmium 3.0 - 15; zinc 0.5 – 2.0; lead is the rest.
The proposed solder allows soldering at a temperature of 260 - 270 C. It spreads well and fills the capillary gap both on galvanized iron and other structural materials. When soldering galvanized iron, the zinc coating is completely preserved and the corrosion properties of the soldered joint are not impaired.
The tensile strength of lap joints soldered with the developed solder is within the range of 7 – 9 kg/lm, and the strength almost does not change when the gap changes.)T
0.05 - to 0.5 mm.
Soldering is carried out equally well with general furnace heating, with burners and a soldering iron using flux based on urea or zinc chloride. The strength of solder joints after corrosion tests in a 3% sea salt solution for 7 months does not decrease; there are no pockets of corrosion
1p is not observed in the soldered seam.
Solder for soldering galvanized iron, containing lead, cadmium, zinc, is distinguished by the fact that, in order to improve the quality of the soldered joint, it contains, %: nickel 0.1 - 0.3 and copper 0.1 - 0 ,2, and the remaining components are taken in the following ratio2i) HHH,%: cadmium 3 0 - 15; zinc 0 5 - 2; lead is the rest.
Soldering - zinc - Great Encyclopedia of Oil and Gas, article, page 1
Soldering - zinc
Zinc soldering is done using zinc-based solders; An aqueous solution of ammonium chloride is used as a flux. Sometimes zinc is soldered with lead-tin solders. Zinc alloys containing more than 2% aluminum are soldered, like aluminum and its alloys, using zinc-tin solders. [1]
It has increased activity when soldering zinc, copper, and nickel. [2]
Hydrochloric acid is used when soldering zinc and galvanized iron, if the zinc layer of the latter is well preserved. [4]
Tin-lead solders containing antimony are unsuitable for soldering zinc and its alloys, since this results in the formation of a very brittle intermetallic compound. The use of antimony in solders allows the use of recycled tin and lead. [5]
However, the presence of antimony impairs the ability of solder to wet the surface of the metal being soldered, and when soldering zinc, brass or a galvanized product, antimony combines with zinc and impairs the strength of the joint. [7]
These solders can be used to solder copper and copper alloys, steel, and iron, but solders with antimony are not recommended for soldering zinc and galvanized iron due to their higher cost. Tin-lead solders are highly technological. [8]
These solders can be used to solder copper and copper alloys, steel, and iron, but solders with antimony are not recommended for soldering zinc and galvanized iron due to their higher cost. Tin-lead solders are highly technological. [9]
These solders can be used to solder copper and copper alloys, steel, and iron, but solders with antimony are not recommended for soldering zinc and galvanized iron due to their higher cost. Tin-lead solders are highly technological. [10]
These solders can be used to solder copper and copper alloys, steel, and iron, but solders with antimony are not recommended for soldering zinc and galvanized iron due to their higher cost. Tin-lead solders are highly technological. [eleven]
POSS-4-6 is used for soldering and tinning of tinplate, iron, brass and copper with rolled or riveted seams and is not suitable for soldering zinc and galvanized iron. [12]
When soldering ornamental steel, tin, bronze and brass, zinc chloride is used as a mordant; when soldering brass, ammonia is used; when soldering zinc and cast iron, hydrochloric acid is used. After mordant with hydrochloric acid, to stop its corrosive effect, the product is washed in a soda solution and finally in clean water. [13]
When soldering ornamental steel, tin, bronze and brass, zinc chloride is used as a mordant, when soldering brass - ammonia, when soldering zinc and cast iron - hydrochloric acid. After mordant with hydrochloric acid, to stop its corrosive effect, the product is first washed (rinsed) in a soda solution and finally in clean water. [14]
Solder grade POSS 4 - 6 is used for soldering and tinning of iron, brass and copper with rolled or riveted seams; it is not suitable for soldering zinc and galvanized iron. [15]
Galvanized iron - Great Encyclopedia of Oil and Gas, article, page 1
Galvanized iron
Galvanized iron is soldered with tin-lead solders with a tin content of 30 - 60% with an acidified solution of zinc chloride. In this case, it is not recommended to use an open flame; you should use a large soldering iron, which is used to lightly rub the soldering area. After soldering, the product must be thoroughly washed to remove flux residues. If the galvanized iron is pre-tinned, soldering can be done with rosin flux, in which case rinsing is not required. When soldering galvanized iron, do not use solders containing antimony. Connections made with such solders are brittle and have little resistance to shock and stress. [1]
Galvanized iron is soldered with tin-lead solders with a tin content of 30 - 60% with an acidified solution of zinc chloride. In this case, it is not recommended to use an open flame; you should use a large soldering iron, which is used to lightly rub the soldering area. After soldering, the product must be thoroughly washed to remove flux residues. If the galvanized iron is pre-tinned, soldering can be done with rosin flux, in which case rinsing is not required. When soldering galvanized iron, do not use solders containing antimony. Connections made with such solders are brittle and have little resistance to shock and stress. [2]
Galvanized iron is soldered with tin-lead solders with a tin content of 30 - 60% with an acidified solution of zinc chloride. In this case, it is not recommended to use an open flame; you should use a large soldering iron, which is used to lightly rub the soldering area. After soldering, the product must be thoroughly washed to remove flux residues. When soldering galvanized iron, do not use solders containing antimony. Connections made with such solders are brittle and have little resistance to impact and stress. [3]
Galvanized iron corrodes quickly in air containing acids (for example, in tunnels) and cannot be used for storing acidic water; Baylis2 states that it should not be used for water with a pH value below 65, since below this limit the formation of soluble zinc salts is possible. The service life of downspouts subject to internal degradation not only by sulfuric acid entrained by rain from the air, but possibly also by corrosive agents resulting from fallen leaves entering the downspout receptacle, as stated by Gardner3, can be greatly extended. coloring. [4]
For example, galvanized iron has good corrosion resistance in rural atmospheres, but is relatively less resistant in urban environments. Lead, on the contrary, is more corrosion resistant in the atmosphere of industrial areas than elsewhere, since a protective film of sulfate forms on its surface. [6]
Joints made of galvanized iron, soldered with tin-lead solders containing antimony, have less strength and ductility than joints made of steel or tinned iron, which is associated with the formation of brittle chemical compounds of zinc with antimony along the boundary of the soldered seam. [7]
A marked sheet of galvanized iron (blank) 7 is placed in the gap between the fixed 1 and movable 2 corners. Then the movable corner 3 with the help of a welded handle 4 bends the sheet at the required angle. [9]
When assessing the properties of tinned and galvanized iron, one should separately evaluate the mechanical properties of the coating from its physical and chemical properties. [10]
When assessing the properties of tinned and galvanized iron, one should separately consider the mechanical properties of the coating and its physical and chemical properties. [eleven]
The valleys are covered with galvanized iron before the roofs are covered with tiles. The connection of roof slopes to stone parapets, firewalls and other walls is carried out by installing grooves in these walls with a depth of V4 bricks and inserting the ends of the tiles into these grooves. [12]
Galvanized iron sheets are laid at the bottom, and wooden gratings are placed on top of it. Products are placed on shelves and racks. Meat carcasses are hung on hooks. The cell doors have locks that ensure their tight fit to the doorway trim. [14]
Pages: 1 2 3 4