Cast iron is one of the main products of ferrous metallurgy; it is not an exaggeration to say that the stability and economic efficiency of various industries, from mechanical engineering to agriculture, depend on the amount of cast iron smelted.
The main raw material for cast iron is iron ore, which consists of iron compounds and so-called waste rock. In addition, the technology for producing cast iron requires the use of additional components. Let's talk about all the materials required for smelting.
Basic materials for blast furnace
1) Iron ores are a source of iron. 2) Coke is a fuel and a reducing agent. 3) Limestone - at high temperatures decomposes to form CaO, which acts as a flux and converts siliceous waste rocks into CaSiO3 slag. 4) Air – supports the combustion of coke with the release of heat. Removes some non-metallic impurities (silicon, arsenic) in the form of volatile oxides. Oxidizes iron oxide FeO in the ore to Fe2O3, which helps preserve iron in the ore. Iron oxide FeO, basic in nature, reacts with SiO2 to form slag FeSiO3. Air makes the ore porous, which promotes uniform reduction of iron.
Components required to produce cast iron.
So, cast iron is a chemically complex substance, therefore, when smelting it, various components are used, each of which performs its own specific function.
On average, to produce 1 ton of metal, about 3 tons (depending on the iron content) of ore, 1.1 tons of coke, 20 tons of water, plus varying amounts of flux are needed.
- The basis of cast iron is metal ore, consisting of various iron compounds, as well as waste rocks. The percentage of Fe in the ore differs depending on the type of material, ranging from 30 to 70%.
- Fluxes, another name for fluxes. Various rocks added to ore during smelting. The main task is to reduce the temperature parameter of ore melting, which ensures more efficient removal of slag. Depending on the type of waste rock, different types of fluxes are used.
- The process of smelting cast iron requires a large amount of thermal energy, and the combustion temperature of the fuel must correspond to the smelting conditions. Coking coal, thermal anthracite, and natural gas are mainly used as fuel in metallurgy.
For a more complete understanding of the melting process, we will consider the properties of these components in more detail.
Minerals in iron ores
The main ore-forming iron minerals are hematite, limonite and magnetite.
Hematite is a red iron ore. Contains iron in the form of anhydrous iron oxide Fe2O3. The iron content in red iron ores is 45-65% with a small amount of harmful impurities.
Limonite is a brown iron ore. Contains iron in the form of aqueous oxides such as nFe2O3×mH2O. Brown iron ore contains 25-50% iron.
Magnetite is a magnetic iron ore. Contains iron mainly in the form of iron oxide Fe3O4, which has magnetic properties. Magnetites - the richest iron ores - contain 40-70% iron.
Raw materials for producing cast iron, preparing them for smelting
The starting materials for producing cast iron in blast furnaces are metal ores, fluxes and fuel.
Ore is a natural mineral raw material containing metals (or their compounds) in quantity and form suitable for their industrial use. Ores are a collection of minerals.
Minerals that contain the necessary metal are called ore, and the rest are called uninhabited rock.
The amount of materials loaded into a blast furnace (ore, fluxes and fuel), calculated in advance in a certain ratio, is called a charge.
Metal ores. The earth's crust contains about 5.1% iron in various chemical compounds.
The most common iron compounds are oxides - compounds of iron with oxygen (of primary importance), sulfides - compounds of iron with sulfur and spars - carbon dioxide compounds of iron.
Red iron ores, brown iron ores, magnetic and spar iron ores are used industrially.
The main ore mineral of red iron ore is hematite - iron oxide (Fe203). The uninhabited rocks are mainly quartz, consisting mainly of (SiOa), and calcite (CaC03), from time to time with clay impurities (A12O3 • 2Si02 • 2H20, etc.).
In the largest deposits of hematite ores, the average iron content is 51-66% (pure hematite contains 70% Fe). The color of the ore ranges from bright red to dark red.
In the USSR, the main deposits of red iron ore are the following. Krivoy Rog deposit - the main basin of the southern metallurgical base, Kursk magnetic anomaly; Atasuskoe and Sokolovsko-Sarbaiskoe fields in Kazakhstan; Korshunovskoye field in Eastern Siberia.
The main ore mineral of brown iron ore is hydrogoethite (limonite) - hydrous iron oxide (Fe2O3/gH20). Uninhabited rocks are of the same nature as in red iron ore.
The iron content in different deposits varies quite widely: from 55 to 30% and lower. Color ranges from brownish-yellow to dark brown.
In the USSR, large deposits of brown iron ore are Kerch, Lisakovskoye and Ayatskoye (in Kazakhstan), Lipetskoye and Tula.
The ore mineral of magnetic iron ore is magnetite - magnetic iron oxide FeO • Fe203 (Fe304). Uninhabited rocks contain silicates (feldspars, granites, etc.), sulfides, calcites, etc. The iron content in rich magnetite ores ranges from 50 to 72%.
The color of magnetite is dark. In the USSR, industrial deposits of magnetite ores are located in the Urals: Magnitnaya, Vysokaya, and Blagodat mountains; in Siberia (Angaro-Pitsky iron ore region) and in other areas.
The ore mineral of spar iron ore is siderite (FeCOg). Spar iron ores occur in marble-like scales of light gray and yellowish-white color; they contain 30-42% iron.
When siderite is fired, carbon dioxide (CO2) is removed • and small pores are formed, which provides easy reducibility during blast furnace smelting. In the USSR, spar iron ores occur near Zlatoust and in the Omutninsky district of the Kirov region.
SG\ According to the seven-year plan, 230-245 million m3 of metal ore will be mined in the USSR in 1965.
Fuel. The fuel used for the blast furnace process must have a high low ash content and calorific value, have porosity, strength at high temperatures, and probably contain less sulfur, which partially passes from the fuel into cast iron and worsens the properties of the latter.
The fuel used in blast furnace production is mainly coal coke and very rarely charcoal.
Fluxes. To separate uninhabited ash and fuel rock, substances called fluxes are introduced into the blast furnace; These substances produce fusible chemical compounds with fuel ash and waste rock that form slag during smelting.
fusibility and composition of slag have a huge impact on the progress of blast furnace composition and smelting of cast iron. In the composition of almost all ores, as well as in coke ash, acidic uninhabited rocks (Si02 + Al2O3) predominate over the main ones (CaO + MgO); Based on this, limestone (containing mainly CaC03) and from time to time dolomite (consisting mainly of CaC03 + MgC03), which produce low-melting compounds with Si02 and Al2O3, are used much more often as fluxes.
Preparation of ores for smelting. For the smelting of cast iron, the ores are subjected to preliminary preparation.
The level of quality of ore preparation for smelting has a huge impact on the progress of smelting, the quality of the metal and fuel consumption.
Separation - crushing large pieces of ore - is carried out by special vehicles - crushers, along with this they try to take pieces of 30-100 mm in size. The fines are sifted out during sorting and screening; they are unsuitable for smelting and are used for sintering.
Washing the ore with water is used to separate the uninhabited rock, which slowly soaks and is carried away by the water.
Ore roasting is carried out to remove water, partially burn and sulfur carbon dioxide, as a result of which the ore is purified and enriched with iron compounds. In addition, the roasting of non-magnetic oxide Fe203 is carried out with the aim of converting it into the magnetic compound Fe304 for the possibility of using magnetic enrichment.
Magnetic enrichment is carried out in devices called magnetic separators. The main part of the separator are electromagnets that serve to form a magnetic field, during ore movement in which non-magnetic particles are separated.
Along with this, magnetic iron oxide (Fe304) is attracted by electromagnets.
Sintering (agglomeration) is carried out for the purpose of agglomerating small powdery blast furnace dust and ore; For sintering, these substances are mixed with crushed fuel. To obtain fluxed agglomerate, crushed limestone is added to the sintering charge, in addition to fuel and ore.
Sintering is carried out at a temperature of 1100-1200° on special sintering belt vehicles, where the fuel burns, as a result of which the composition of the charge changes: limestone at a temperature of about 900° decomposes into calcium oxide (CaO) and carbon dioxide (CO2), sulfur burns out, iron oxide (Fe203) is partially reduced to oxide (FeO), which with SiOa of uninhabited rock forms iron silicate Fe2Si04. This silicate melts and binds other particles of the charge, along with this, porous sintered pieces of material called agglomerate are formed.
To increase productivity, fluxed (self-melting) agglomerate is introduced into the blast furnace charge composition.
When working on fluxed sinter, the consumption of coke and fluxes is reduced and the productivity of the furnaces is increased.
Each source material is loaded into the blast furnace in separate portions; these portions are called ears.
How steel is hardened. Iron production
You read the article, but didn't read the magazine...
- Selection of materials for making passive coatings
- Materials for work at elevated temperatures
- Materials for making soldering iron tips
- Materials used for the manufacture of cutters
Preparation of ore for iron production
For normal operation of a blast furnace, it must be loaded with lump material of optimal size. Too large pieces of ore and other materials will not have time to react properly, and some of the material will be useless. Pieces that are too small fit too tightly together, without leaving the necessary passages for gases to pass through, making the oven difficult to operate.
The optimal size of charge pieces is 30-80 mm. Larger pieces are crushed to the optimal size.
On the other hand, when crushing materials and mining ore, along with large pieces, fines are formed, which are also not suitable for smelting. Such materials are agglomerated to the required size using agglomeration and rolling methods.
In addition to agglomeration and rolling, ore is enriched. Enrichment is the pre-processing of ore without changing the chemical composition of the main minerals and their state of aggregation. Ore beneficiation is carried out to increase the iron content in it. In this case, a significant part of the waste rock is removed from the ore. When beneficiating ores, various methods are used: ore washing, flotation method, gravity method and magnetic enrichment.
Iron Age
Historians say that it was much easier to extract iron than copper or tin. The thing is that it is found in the form of oxide and nitrous oxide everywhere. So why didn't people start using iron sooner? The answer is simple: the production of this metal is an incredibly complex and labor-intensive process, taking place in several stages. It took more than one century of development to study this process. Therefore, it is not surprising that the metallurgists of those times were popularly considered to be real sorcerers who burned magical things.
Blast furnace smelting
Blast furnace smelting consists of separately loading fluxed sinter and coke into the upper part of the furnace (top). They are placed in the oven in layers. The charge is heated by the heat of combustion of coke in hot air, which is blown into the lower part of the blast furnace. The charge gradually falls down. As a result of the physicochemical interaction of the charge components and rising gases, two immiscible liquid layers are formed in the lower part of the furnace - the forge - cast iron on the hearth flank and slag above the cast iron.
Liquid cast iron is released every 2-3 hours, in large furnaces - every hour. The slag from the furnace is released along with the cast iron. They are separated using special shutters.
A blast furnace usually operates continuously for several years – up to 10 years.
The first mentions of cast iron
Today, China is considered the country where cast iron production began. Historians say that this happened around the fifth century BC. In the Celestial Empire, coins, household items and various weapons made from cast iron were extremely popular. Many cast iron castings have survived to this day, for example, a magnificent cast iron lion, whose height is 6 meters and length is 5. Scientists have proven that this statue was cast in one go, which undoubtedly testifies to the great skill of the first Chinese metallurgists.
Interesting fact: all over the world, the beginning of the production of malleable cast iron is considered to be the 19th century AD, although it is reliably known that in China swords were made from it even before the birth of Christ!
Fuel combustion and formation of reducing agents
Combustion of fuel carbon occurs in the lower part of the furnace when air interacts at a temperature of 1000-1300 ºС with coke:
C + O2 = CO2.
The resulting carbon dioxide rises to the hot coke and reacts with it to form the reducing agent CO
:
CO2 + C = 2CO.
The reducing agent CO in the presence of iron decomposes by reaction to form the atomic soot reducing agent C
:
2CO = C + CO2.
The origins of production in Russia
When did cast iron production begin in Russia? Archaeological excavations carried out on the territory of large cities of the Golden Horde prove: the emergence and development of this production in Russia began during the Tatar-Mongol yoke! The proximity of the Mongol kingdom to China played a certain role in this.
Almost all Tatar-Mongolian cities were inhabited by Russians, who had their own workshops and shopping arcades here. They not only adopted the knowledge of local masters, but also shared their own. After the Horde fell, technology continued to develop and improve. Already in the 16th century, under Vasily the Third and Ivan the Terrible, the production of cast iron began to be actively used in artillery, mainly cannonballs and small cannons were made from it. At the same time, historians say, cast iron was also used in the casting of bells. The main production took place in cities such as Moscow and Tula. It is worth noting that until the 17th century, Europe did not know such technologies, and therefore Russian factories could actively export various tools and cannonballs made of cast iron to European countries.
Reduction of iron oxides
The main task of the blast furnace process is the reduction of iron from its oxides. The main role in the reduction of iron is played by carbon monoxide and atomic black carbon, which are formed as a result of the blast furnace process.
The reduction reaction zones and their temperatures in a blast furnace are shown in Figure 2.
Figure 2 – Scheme for the reduction of iron oxides during the production of cast iron in a blast furnace
The reduction of iron oxides occurs in the following sequence:
Fe2O3 → Fe3O4 → FeO → Fe
Main recovery reactions
are the following:
Fe2O3 + 3C = 2 Fe
+ CO 3Fe2O3 + CO = 2Fe3O4 + CO2Fe3O4 + CO = 3FeO + CO2FeO + CO =
Fe
+CO2
Hydrogen, which is formed from water contained in the charge, also participates in the reduction of iron.
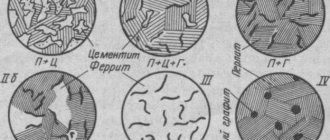
Cast iron classification
There are different principles according to which cast iron is classified. To understand some of the information, special training in metallurgy is required. The rest is clear to everyone. The main indicator of the main classification is the content and state of carbon in the alloy.
- In white cast iron this element is in the form of carbide. The mass fraction of iron exceeds 3%. The alloy is characterized by high brittleness and is used mainly after alloying.
- The gray modification contains carbon plates. The product has high resistance to friction.
- The malleable modification includes carbon flakes. The production of this type of cast iron is difficult, so the alloy is more expensive and is used for the manufacture of particularly important parts.
The operational capabilities of a metal product are determined by its specific qualities:
- wear resistance;
- resistance to friction;
- inertness to corrosion;
- heat resistance;
- no reaction to the magnet.
According to the characteristics given, cast iron is divided into groups. In addition, alloys are classified by hardness, tensile strength, and other physical parameters.
Production technology
The blast furnace process is a set of mechanical, physical and chemical-physical processes that occur in a functioning blast furnace. The loaded fluxes, ores and coke are converted into cast iron during the smelting process. From a chemical point of view, this is a redox process. Essentially, iron is reduced from oxides, and reducing agents are oxidized. But the process is usually called reduction, since the ultimate goal is to obtain metal.
The main unit for implementing the smelting process is the furnace (shaft). It is extremely important to ensure the counter-movement of the charge materials, as well as their interaction with the gases that are formed during smelting. To improve the combustion process, an additional supply of oxygen, natural gas and water vapor is used, which together is called blast.
Types of cast iron
There are only two types of cast iron: white and gray. The difference between them lies in the chemical composition and heat treatment process. Thus, white cast irons are the result of very rapid cooling, while gray cast irons are the result of slow cooling. Whites are characterized by qualities such as fragility and hardness. They are extremely difficult to cut; in the process, pieces break off from them. Therefore, white cast iron is used only as blanks for the production of other grades of cast iron. For example, as a result of firing of this type, malleable cast iron is obtained. Please note: the name "malleable" has nothing to do with the forging process. According to historians, it appeared due to the fact that horseshoes with such characteristics were previously made from cast iron. This type is actively used in agricultural engineering and the automotive industry. The main difference between gray cast iron is ductility, combined with high strength. This allows them to be used in areas such as machine tool building, agriculture and the automotive industry, and household use.
By the way, there are so-called half cast irons. They have intermediate properties of the white and gray species. In addition, by adjusting the cooling intensity of a given alloy, it is possible to obtain a variety of castings that will differ in strength, ductility and other properties. Cast irons with special properties include:
- antifriction, used for the manufacture of bushings, shafts, bearings;
- wear-resistant, necessary for the creation of pumping equipment, various parts for the nitrogen industry, furnace casting;
- heat-resistant, which are used in the manufacture of furnace castings, pipe complexes and gas turbine engines;
- heat-resistant, suitable for making fittings and boiler parts from kilns;
- resistant to corrosion, indispensable for the manufacture of various parts in the chemical and aviation industries, which are used in aggressive environments.
Properties and purpose
The most commonly used alloying elements are nickel, manganese, chromium, silicon, lead, selenium and boron. Less commonly used are aluminum, copper, niobium, zirconium and tungsten. The purposes of these elements are very diverse, and when used in the right proportions, steels are obtained with certain characteristics, which, however, cannot be achieved with ordinary carbon steels. Alloys are usually classified based on the elements , the content of which is the highest, and which are called basic components. Elements that are found in smaller proportions are considered secondary components.
Iron itself is not particularly strong, but its strength increases significantly when it is alloyed with carbon and then quickly cooled to produce steel. Some characteristics of steel - soft, semi-soft, semi-hard, hard - are largely determined by the carbon content, which can range from 0.10 to 1.15%.
Risks
Some ferroalloys are produced and used in fine particle form; Airborne dust poses potential toxicity, fire and explosion hazards. In addition, occupational exposure to fumes from the manufacture of certain alloys can lead to serious health problems. A number of tin alloys are hazardous to health (especially at high temperatures) due to the harmful properties of the metals with which tin can be alloyed (for example, lead).
Practical application of alloying additives
Nickel, osmium, ruthenium, copper, gold, silver and iridium are alloyed with platinum to increase hardness. Alloys formed with cobalt have gained importance due to their ferromagnetic properties. Rhodium is used as an anti-corrosion electrolytic coating to protect silver from tarnishing. Rhodium is alloyed with platinum and palladium to make very hard alloys. The purpose of alloying with copper is to increase corrosion resistance. Silver is also alloyed with copper. In its pure form, silver is too soft for coins, cutlery and jewelry; for all applications, it is hardened by alloying with copper.
Ferrous alloys
Ferrous alloys are iron and its alloys. The significant carbon content makes cast iron very brittle. Despite their brittleness and lower mechanical properties than steel, their low cost, ease of casting and specific characteristics make them one of the world's most valuable products with the largest production tonnage.
Non-ferrous alloys
Non-ferrous alloys are alloys that do not contain iron or contain relatively small amounts of iron. Their characteristics are significant corrosion resistance, high electrical and thermal conductivity, low density and ease of production.
Stainless steel
The general characteristics of stainless steel make it a universal material that adapts well to the requirements of today. Any type of alloy has its own advantages depending on the chemical composition.
Aesthetics. There are a number of surface finishes available, from matte to glossy, satin to engraved. Finishes can also be patterned or painted, making stainless steel a unique and aesthetically pleasing material. Architects often choose this material for construction work, interior design and urban furniture.
https://youtube.com/watch?v=zEpXGAB98hM
Mechanical properties: Stainless steel has better mechanical properties at room temperature compared to other materials, which is an advantage in the construction sector as it allows for weight savings per m² or smaller dimensions of structural elements. Good elasticity and hardness combined with good wear resistance (friction, abrasion, impact, elasticity...) allow stainless steel to be used in a wide range of projects. In addition, stainless steel can be installed on a construction site, despite winter temperatures, without the risk of brittleness or breakage, which does not prevent construction time from being extended.
Fire resistance. Compared to other metals, stainless steel has better fire resistance in construction due to its high melting point (above 800 °C). Stainless steel does not emit toxic fumes. Corrosion Resistance: With a chromium content of 10.5%, stainless steel is permanently protected by a passive layer of chromium oxide that forms naturally on its surface when exposed to air humidity. If the surface is damaged, the passive layer is restored. This provides corrosion resistance.
Characteristics of cast iron
High-quality cast iron is characterized by the following qualities:
- excellent heat capacity;
- good resistance to corrosion;
- increased heat resistance.
These and other characteristics make it possible to use cast iron both in everyday life and in heavy industry. Russian-made cast iron cookware is especially popular. Not only frying pans and pots are made from this material; fondues, frying pans, baking dishes, stewpans and grills are also found.
It is worth noting that dishes made from this material are equally well suited for frying pancakes, preparing stews, porridges, and simmering pilaf. The fact is that cast iron heats up quite slowly, but it perfectly accumulates heat and distributes it evenly. Experts say: different companies are engaged in the production of cast iron cookware. They all produce products of approximately the same quality. Of all this diversity, St. Petersburg products stand out. This plant is one of the largest manufacturers of cast iron cookware in the Russian Federation.
Cast iron parameters
Density - 7.2 g/cm3. The melting point is 1200 °C. The fragility and low ductility of the alloy is due to the following factors:
- Increased bond length between Fe atoms due to increased carbon content;
- Incomplete introduction of carbon atoms into the structure of the iron matrix due to the low melting point compared to steel.
It is for these reasons that this solid metal solution has found wide application in the production of parts with high strength. However, it is not suitable for products subject to loads that change rapidly over time.