Historical information
The chemical element has been known to people since ancient times. One of the first methods of metal extraction mastered by man was the smelting of lead. The first archaeological finds confirming this were lead beads found from the times of Çatalhöyük (modern Turkey). The items date back to 6400 BC.
The oldest lead figurine of a girl in long clothes was excavated in Egypt. It dates back to the time of the first dynasty of the pharaohs (3000 BC).
Lead pipes made up the ancient Roman water supply system. In the Ancient Roman Empire, up to 80 thousand tons of this metal were smelted annually. In Rus', since ancient times, lead has been used as roofing for cathedrals and churches.
Since time immemorial, the low melting point of lead has made it possible to obtain the metal and manufacture products of any shape from it.
Note! Over the course of 20 years, the Industrial Revolution since 1840 has raised the volume of annual lead smelting in the world from 100 to 250 thousand tons per year.
APPLICATION
Lead nitrate is used to produce powerful mixed explosives. Lead azide is used as the most widely used detonator (initiating explosive). Lead perchlorate is used to prepare a heavy liquid (density 2.6 g/cm³) used in flotation beneficiation of ores, and it is sometimes used in high-power mixed explosives as an oxidizing agent. Lead fluoride alone, as well as together with bismuth, copper, and silver fluoride, is used as a cathode material in chemical current sources.
Lead bismuthate, lead sulfide PbS, lead iodide are used as cathode material in lithium batteries. Lead chloride PbCl2 as a cathode material in backup current sources. Lead telluride PbTe is widely used as a thermoelectric material (thermo-emf 350 µV/K), the most widely used material in the production of thermoelectric generators and thermoelectric refrigerators. Lead dioxide PbO2 is widely used not only in lead batteries, but also on its basis many reserve chemical current sources are produced, for example, lead-chlorine cell, lead-fluorescent cell and others.
Lead white, basic carbonate Pb(OH)2•PbCO3, dense white powder, is obtained from lead in air under the influence of carbon dioxide and acetic acid. The use of white lead as a coloring pigment is no longer as common as it once was due to its decomposition by hydrogen sulfide H2S. Lead white is also used for the production of putty, in the technology of cement and lead carbonate paper.
Lead arsenate and arsenite are used in insecticide technology to kill agricultural pests (gypsy moth and cotton boll weevil).
Lead borate Pb(BO2)2•H2O, an insoluble white powder, is used to dry paintings and varnishes, and, along with other metals, as coatings on glass and porcelain.
Lead chloride PbCl2, a white crystalline powder, is soluble in hot water, solutions of other chlorides and especially ammonium chloride NH4Cl. It is used to prepare ointments for treating tumors.
Lead chromate PbCrO4 is known as chrome yellow dye and is an important pigment for making paints, for dyeing porcelain and fabrics. In industry, chromate is used mainly in the production of yellow pigments.
Lead nitrate Pb(NO3)2 is a white crystalline substance, highly soluble in water. This is a binder of limited use. In industry, it is used in matchmaking, textile dyeing and printing, antler dyeing and engraving.
Since lead absorbs γ radiation well, it is used for radiation protection in X-ray facilities and in nuclear reactors. In addition, lead is considered as a coolant in projects of advanced fast neutron nuclear reactors.
Lead alloys are widely used. Pewter (tin-lead alloy), containing 85–90% Sn and 15–10% Pb, is moldable, inexpensive, and used in the manufacture of household utensils. Solder containing 67% Pb and 33% Sn is used in electrical engineering. Alloys of lead and antimony are used in the production of bullets and typographic fonts, and alloys of lead, antimony and tin are used for figured casting and bearings. Lead-antimony alloys are commonly used for cable sheaths and electric battery plates. There was a time when cable sheaths used a significant portion of the world's lead production, due to the good moisture-proof properties of such products. However, lead was subsequently largely replaced from this area by aluminum and polymers. Thus, in Western countries, the use of lead on cable sheaths fell from 342 thousand tons in 1976 to 51 thousand tons in 2002. Lead compounds are used in the production of dyes, paints, insecticides, glass products and as an additive to gasoline in the form of tetraethyl lead (C2H5)4Pb (a moderately volatile liquid, the vapors of which in small concentrations have a sweetish fruity odor, in large concentrations - an unpleasant odor; Tm = 130 ° C, Bp = +80 °C/13 mm Hg; density 1.650 g/cm³; nD2v = 1.5198; insoluble in water, miscible with organic solvents; highly toxic, easily penetrates skin; MPC = 0.005 mg/ m³; LD50 = 12.7 mg/kg (rat, oral)) to increase octane number.
Read also: How to properly nail lining horizontally
Used to protect patients from radiation from X-ray machines.
Melting lead is not difficult. This can be done even at home using a suitable thin-walled steel or even aluminum container. The melting point of lead is low. To achieve it, a flame from a burner on a household gas or electric stove is sufficient.
Being in nature
Soldering aluminum
Plumbum is usually not found in its pure form. It is found in more than 100 different minerals in the form of intermetallic agglomerates. Lead is present in uranium and thorium veins. Large accumulations of lead-zinc ores have been discovered and are being developed in Transbaikalia and the Primorsky region. Lead is mined in various deposits in the Urals and Norilsk.
The largest deposit with a high content of lead is located in the uranium ores of the Kohistan Ladakh arc (northern Pakistan).
Fossil lead
ORIGIN
It forms impregnations in igneous, mainly acidic rocks; in deposits of Fe and Mn it is associated with magnetite and hausmannite. Found in placers with native Au, Pt, Os, Ir.
Under natural conditions, it often forms large deposits of lead-zinc or polymetallic ores of the stratiform type (Kholodninskoye, Transbaikalia), as well as skarn (Dalnegorskoye (formerly Tetyukhinskoye), Primorye; Broken Hill in Australia) type; galena is often found in deposits of other metals: pyrite-polymetallic (Southern and Middle Urals), copper-nickel (Norilsk), uranium (Kazakhstan), gold ore, etc. Sulfosalts are usually found in low-temperature hydrothermal deposits with antimony, arsenic, and also in gold deposits (Darasun, Transbaikalia). Lead minerals of the sulfide type have a hydrothermal genesis, minerals of the oxide type are common in weathering crusts (oxidation zones) of lead-zinc deposits. Lead is present in clarke concentrations in almost all rocks. The only place on earth where rocks contain more lead than uranium is the Kohistan-Ladakh arc in northern Pakistan.
Read also: Stone for sharpening knives
Receipt
How to solder a wire without a soldering iron
The raw materials for lead extraction are rocks that include helenite. The heavy metal smelting process consists of several phases. From the initial raw material, a concentrate containing from 40 to 70 percent plumbum is isolated by flotation. Next, manufacturers take different paths.
One of the ways to transform the product into werkbley (blank lead) is smelting using the regeneration method. Another method is that the metal is restored from the oxide by melting the raw material in a water jacket heater.
The resulting werkley containing 90% lead is purified from copper. Then arsenic and antimony are removed by alkaline refining. Then silver and zinc are isolated. The effects of magnesium and calcium exclude bismuth. As a result, lead with a purity of 99.8% is obtained.
Global lead production based on research by international organizations for 2005
| Manufacturer country | Volume, kilotons |
| Countries of Europe | 2220 |
| China | 1430 |
| Russian Federation | 1120 |
| South Korea | 650 |
| Kazakhstan | 570 |
| Ukraine | 410 |
Melting in artisanal conditions
- Lead, whose melting point is low, allows its use for casting a variety of crafts and sinkers for fishing at home. Forming a melt is not difficult, but basic safety and care must be observed.
- Metal melting should be carried out in a well-ventilated area. You can choose a hand burner as a heat source, and use a container made of a more durable and heat-resistant metal as a vessel.
- Having placed the material in the heating container, turn on the heat source at maximum power and direct the temperature flow closer to the material being melted. It will take some time to convert a significant amount of raw material into liquid.
- After turning off the burner, the molten material can be poured into the prepared casting mold. Wearing special gloves, carefully pick up the container with liquid, gently rotating it to prevent the formation of bubbles.
- It is necessary to pour metal into the mold at a distance so as not to burn exposed parts of the body with hot lead fumes. After pouring, leave the mold to cool to a safe temperature.
- Spilled melt can be easily mechanically removed from the surface using a screwdriver or chisel and used for the next melt.
- The material mixes well with other metals, which affects the composition and quality of the casting. When working, it is necessary to use special clothing and thick gloves to protect the skin of your hands from metal dust.
- Before pouring, you need to make sure that the mold is completely dry. In the presence of moisture, instantaneous evaporation may occur, which will result in the contact of the melt on the body.
Read also: Stone sawing machine
Technological properties and characteristics
The characteristics of the metal can be represented by the list:
- Lead density and mass;
- Lead smelting temperature;
- Mechanical properties;
- Corrosion resistance.
Lead density and mass
The density of the metal is 11342 kg/m3. This means that a metric cube of lead weighs 11.342 tons. Its large specific gravity allows it to be used as payloads in various devices.
Lead smelting temperature
Molten metal in its pure form has a temperature of about 400 degrees. In this state, lead has fluid properties. The casting qualities make it possible to pour lead in a liquid state into molds of complex configurations.
Pouring a mold with lead
The metal boils when heated to 1750 degrees. During boiling, volatile fumes arise in the form of lead dust and oxide vapors, which can cause severe poisoning to the human body.
Mechanical properties
The chemical element has softness and ductility, which allows cold rolling to achieve the state of thin foil. Cold deformation does not affect the change in mechanical properties.
Corrosion resistance
The chemical inertness of the element is close to that of noble metals. In the air, plumbum practically does not corrode. The rapidly forming oxide film on the surface of lead puts an insurmountable barrier to corrosion processes.
Aggressive environments for lead are hydrogen sulfide, coal anhydrite and sulfuric acid. Under their influence, the metal is actively destroyed.
Characteristics and features of lead
The metal is dirty silver or silvery-bluish in color and has a high specific gravity - 11.34 g/cm 3 . Tensile strength no more than 18 MPa. for compression within 50 MPa. Due to its high ductility, it lends itself to many types of mechanical processing; it is cold rolled to the state of foil, stamped, cut, and cold-fretted. When drawn, lead breaks; wire is made from it by pressing the blanks in dies.
Read also: Requirements for the condition of a turner’s work clothes
When cooled to -266°C, lead becomes a superconductor, despite its low electrical conductivity at room temperature (resistivity 0.22 Ohm * mm 2 /m). In air, lead quickly fades when cut, passes, and becomes covered with an oxide film characterized by low chemical activity. The metal itself is also resistant to corrosion damage, so acid-resistant vessels and containers are made from lead and alloys. Pb is the last element on the periodic table with stable isotopes. The metal is capable of retaining radioactive radiation.
The cubic, face-centered structural lattice ensures the toughness of the metal; it is difficult to break, but it cuts well and is easy to scratch and crush. When heated, the structure becomes fluid and the initial viscosity decreases.
Application areas of lead alloys
Lead compounds are divided into high-alloy and low-alloy alloys. The former are formed by adding a large number of chemical elements that provide high strength, abrasion resistance and low shrinkage at a lower melting point.
Low alloy lead compounds are produced by small inclusions of substances such as tin, antimony, copper and cadmium. This achieves increased resistance of the alloy to corrosion processes in a polluted atmosphere, inorganic acidic environment.
The alloys are used in acid and alkaline batteries, as shells for both high-power and low-voltage cables. Compounds of antimony or copper with lead are used for the production of pipelines, sheet lining of various devices and protective mats against radiation damage.
Melting process
The following is used as a heat source for melting scrap:
- a fire, with a smelter stand placed above it;
- blowtorch, it is fixed in a stationary position;
- a gas burner, with which the metal is heated both from below and from above, alternately;
- kitchen stove (gas or electric).
The container is installed so that the flames do not extend beyond the bottom area.
Lead smelting begins with a preparatory stage: you need to prepare a smelting container and grind the scrap. It is cleaned of impurities, possible moisture, and contaminants. Then cut into small pieces with a knife or metal scissors. It is difficult to break lead pieces; they bend well. The smaller the scrap, the faster it will melt. It is recommended to gradually add it to the melting tank. When the pieces are loaded into the melt, the risk of the melt overheating to the volatility point is reduced. You should not heat the pieces to a reddish tint, this is a signal that they are forming toxic volatile compounds.
If a container with thick walls is used for the melt, it is preheated. Dry the tin enough. The melting pot is filled no more than half, preferably 1/3. A thick layer will not heat up evenly.
Read also: Capillary viscometer operating principle
The melting pot must stand firmly on the burner and not wobble. After the scrap melts, a slag cap forms on the surface. It is removed before pouring the melt into the mold. It is preheated so that there is no sharp temperature contrast. The melt is poured unevenly into a cold mold. Potholes, folds, and other defects form on the casting surface.
Home and industrial methods
Without tin-lead solders (PLS), the existence of such an industry as radio engineering is impossible. Many industrial products contain POS coatings.
Tin-lead solders
The industry supplies the market with solder product:
- cast pigs;
- wire;
- foil tape;
- solder tubes with flux;
- powder or paste.
Alloys containing 90% tin and 10% lead are used for soldering products, which are then electroplated with gold or silver. The melting point of pure tin is 2310 C. Therefore, the solder will melt when heated to 2200 C.
Tubular solder with flux
Tin-lead POS with a predominance of tin in its composition (61%) has a lower melting point - 191%. POS 61 is used to cover contact groups in various devices; it is also used to process thin wire for windings of electric motor armatures and transformer coils.
Important! Taking into account the temperature at which tin melts, the % lead content in the alloy is regulated. This achieves a comfortable temperature regime, at which the tin-lead solder quickly turns into a liquid state.
POS 30 melts at 256 degrees. The compounds are less durable than products with a higher tin content.
10 percent solder is far from the temperature threshold at which tin melts. Therefore, POS 10 is used as a durable material for tinning large metal surfaces.
Melt preparation and pouring
In industrial conditions, the melt is prepared in special crucibles, which are placed in electric furnaces (equipment equipped with electronic measuring equipment that maintains the desired melting mode).
In radio engineering production, special heating baths are used to prepare solder for printed circuit boards of radio circuits.
In workshops and at home, solder is melted with a soldering iron tip. To prepare a large volume of molten metal, it is placed in a copper vessel on an electric stove. The alloy in the form of scrap is loaded into the melting bath gradually, as the next layer of metal melts.
Fishing varnishes
Avid fishermen at home cast fishing sinkers and spoons by pouring molten tin into clay molds. The spoons are then coated with waterproof varnishes.
Interesting. Fishing varnish is used to protect against the appearance of oxides on various figurines and other products.
Fishing varnish
Melting at home: preparation
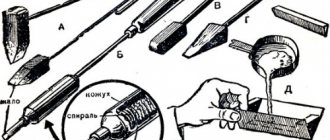
It is necessary to select a suitable container; it is desirable that its handle is made of heat-resistant material. An old teapot, coffee pot or other disused kitchen utensil with a comfortable handle is good for these purposes. You can also melt the material in an old cast iron pan, and use a deep spoon with a long handle to pour it.
Read also: Voltage stabilizer 7805 characteristics
In a pinch, a tin can will do. But here you can’t do without pliers. They can be used to remove heated dishes from the heat and pour molten lead into the prepared mold. But this should be done very carefully. For convenience, it is better to make a groove on the rim of the tin. Then the molten metal will flow out in a thin stream without smudges and exactly in the right place. The place for gripping with pliers should also be equipped in advance so as not to perform unnecessary manipulations with dishes and heated metal.
The lead, prepared and purified as much as possible from foreign impurities, is crushed into small pieces if possible. This way it will melt faster. The container is securely installed above the burner and warmed up. This is done to burn out moisture and unwanted impurities from its surface.
Methods for getting rid of oxide
When exposed to air, lead products become covered with an oxide film. This is the result of the ionic interaction of oxygen and lead atoms. The oxide becomes not only protection against an aggressive environment, but also a barrier to electric current.
Important! Mechanical cleaning will not bring the desired result. The film will recover quite quickly. Sunflower oil, graphite grease or varnish can help get rid of oxides.
At home, the product is placed in a vessel with sunflower oil for about five minutes. After which it is removed from the vessel and allowed to dry.
In industrial conditions, graphite lubricant is used. The lead surface treated with the product retains its shiny appearance for a long time.
Preparing to smelt lead
The technology for producing lead from ore in industrial conditions consists of the following stages:
- Preliminary enrichment of ore by flotation (the conglomerate contains up to 70% pure lead).
- Obtaining rough raw materials by reduction smelting or processing in a liquid bath using Vanyukov technology.
- Further purification of a composition containing up to 90% lead from impurities using sulfur, alkalis and zinc foam.
- Shipments to customers of finished metal containing up to 0.2% impurities.
In domestic conditions, it is only possible to melt lead scrap (for example, battery plates washed from electrolyte residues). At the preparation stage, you should clear the work area of flammable objects and wear protective clothing made of fabric that does not absorb melts.
It is recommended to provide a protective mask, and to carry out all work outdoors or in a well-ventilated area.
Melting container
Since domestic conditions do not place high demands on the cleaning of the material or the stability of the heating temperature, a metal can with a groove for draining the melt is used for melting. To hold the container over the burner or for installation and removal from the electric stove, a handle with wooden pads is attached to the side surface with rivets. You can use pliers instead, but there should be a place on the side of the container for a comfortable and secure grip.
At home, a metal can with a groove is used for melting.
Necessary materials
To perform work in domestic conditions you will need:
- gas or electric stove installed under an exhaust hood;
- tin container with a capacity of 100-200 ml;
- pliers and wire cutters;
- casting shape;
- a sheet of plywood for laying a mold or crucible;
- protective gloves.
Interesting: Review of the best thermal metal cutting machines