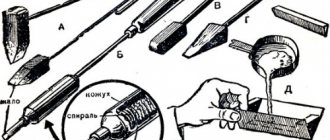
How to treat parts with acid
Liquid fluxes are applied to surfaces with a brush. In this case, a more accurate and uniform wetting of the elements to be soldered is ensured, so the brush should be included in any soldering kit. At the same time, as already written above, both more active fluxes and less active ones, to one degree or another, have a destructive effect on both the connected surfaces and the solder. If the flux is not removed at the end of the work, then on steel parts, for example, rusting processes will occur at a much faster pace.
To prevent this, after soldering, parts coated with flux must be treated with neutralizers. The simplest of them is water. To remove F-38 N, you don’t need to use anything other than it. Soda neutralizes well the effects of hydrochloric, orthophosphoric, and acetylsalicylic acid, as it is a base. After soldering, a soda solution should be applied to the parts, which is then washed off with water. Residues of VTS are removed with alcohol or acetone.
Non-standard methods
If there is no rosin nearby for soldering, you can use material for rubbing bows.
It's better cleaned. All properties are saved. The cost of replacement will be significant. Savvy craftsmen who have solders with flux suggest soaking it in alcohol and waiting until the rosin has completely dissolved. It takes a little time.
They say that such an alcoholic extract can successfully replace rosin. The alcohol component will gradually evaporate. The solid component meets the requirements for fluxes.
When working with old equipment, you can replace rosin with residues in places of old soldering. You need to touch the wire and soldering iron there and make a connection. This method is acceptable for extreme situations with not very high requirements for seams.
Types of soldering acids and application features
Soldering acid is divided into two main types, regardless of the scope of application, orthophosphoric and hydrochloric type. Regardless of the composition, the purpose is to remove oxides and contaminants from soldering areas. A high-quality, neat seam can only be made if the metal preparation conditions are met. The durability of materials is increased due to the formation of a protective film against oxidation on the joint surface.
It is important to know that using flux when working with electronic boards is strictly prohibited. Thin and fragile elements can be erased from the board structure; soldering acid produces conductive connections. All these factors can have a detrimental effect on the performance of the unit and the overall condition of the structure.
Zinc chloride flux
A solution of zinc chloride is used for soldering iron compounds. The composition is zinc dissolved in hydrochloric acid. The solution is made as follows:
- granular zinc is prepared;
- depending on the technical specifications, add a solution or concentrate;
- After the chemical reaction of zinc, it is possible to use a mixture.
Proportional parts are taken using the example of 1 liter of saline solution per 400 grams of granulated zinc. At the end of the work, the surface should be treated to stop the reaction; a soap solution is excellent for this. Before making it yourself, you should remember that it is important to follow the sequence. The acid is diluted with zinc, and gases are formed, resulting in a rather explosive mixture. All actions are carried out in a ventilated place.
Oleic acid
Olein is excellent for soldering aluminum alloys. Not used in its pure form, available only in technical condition. A stable state is achieved by mixing olein with various fatty acids. Next, lithium iodide reacts, which completes the mixing of the aluminum soldering mass.
Electric soldering iron
To tin the wire efficiently, you need to adhere to the following recommendations:
- Preparing the soldering iron. To sharpen the tip, you must use sandpaper. The craftsman should achieve a perfectly smooth and shiny surface of the product. The hot tip is placed in flux and solder. The tip is applied to a small wooden plank. The manipulations are repeated exactly until the product takes on the desired appearance.
- Wire processing. They must be cleared of the braid (at a distance of 1.5-2 cm from the edge) and coated with prepared flux. Place the soldering iron tip on top. Only after melting can the wire be removed.
- Final works. The tip of the tool is treated with solder, and the required area is heated to the optimal temperature. After covering the wires with tin, you need to avoid unnecessary movements. To speed up cooling, you can turn on the fan.
Features of application and soldering with soldering acid
The category into which soldering acid falls differs from other reagents and has a number of positive properties. As a flux, the product is distributed only in liquid form; some compositions can be diluted to reduce the concentration when interacting with metal. Before using the element, it is worth understanding what soldering acid is needed for.
Before soldering metals, it is necessary to prepare the areas for use. With prolonged use, metals tend to oxidize, and a layer of dirt and dust forms on them. If it is possible to deal with dirt mechanically, using sandpaper or a file, then oxides can only be removed using chemical solutions. Soldering acid helps prevent the formation of a new film and remove existing deposits.
Cleaning metal with soldering acid
Basic metals that can be treated with soldering acid:
- copper alloys of any proportions;
- iron products;
- nickel;
- all kinds of non-ferrous metal alloys;
- steel.
Brass and copper alloys can be soldered using borax. Aluminum or steel products will not be connected in any way without soldering acid. Before soldering with acid, the part is treated to remove solid deposits, and after soldering it is washed off with water with a low alkaline content. Varieties of soldering products are produced according to GOST 23178-78 standards, they have fluidity and low viscosity.
Acids with stearin
It happens that there is nothing suitable for replacement nearby.
Then any acid except concentrated sulfuric and nitric acid is suitable for removing oxides. To prevent oxidation of the metal, you can thoroughly clean it and the solder, then coat them with stearin. Stearin (paraffin) can be taken from candles.
Before applying a protective layer to the metal, stearin must be melted without overheating. The protective layer will prevent contact with oxygen. Soldering should be carried out under the stearin layer.
Some craftsmen, in order to replace rosin, spread the well-known BF-6 glue onto the cleaned metal surface. In this case, you need to solder while pressing the soldering iron firmly.
The above methods are a “first aid” for a solderer. In unexpected situations, a way out can always be found using simple and accessible means. But still, for the quality of soldering, it is better to use special tools.
Phosphoric acid flux
Another common soldering acid is phosphoric acid (H3PO4). It successfully removes oxides from metal surfaces and protects them from the formation of new compounds with oxygen, which form a film on the metal that prevents parts from soldering. It is no coincidence that phosphoric acid is included in most products for anti-corrosion treatment of steel structures.
For soldering chromium and nickel alloys, acid is not used in its pure form. Almost 1/3 of the flux consists of ethyl alcohol. H3PO4 accounts for 32%, and rosin accounts for 6% of the composition. In other compositions for tinning and soldering, the volume of acid can reach almost 100%. Often, orthophosphoric acid is diluted together with zinc chloride, the mass content of which in the flux can range from 50% to thousandths of a percent. H3PO4 is used not only for joining parts made of nickel alloys, it is used for soldering products made of low-alloy steel and pure copper or its alloys.
Table of acid fluxes.
Orthophosphoric acid is part of the F-38 N active flux, which is used for soldering:
- alloy, low-carbon and medium-carbon steel;
- copper and its alloys;
- chromium-nickel alloys.
F-38 N is used for soldering in places with difficult access and protects the soldered parts from corrosion. It contains:
- diethylamine hydrochloride;
- H3PO4 (25%).
Phosphoric acid is explosion- and fireproof, but working with it and storing it must be carried out in compliance with all safety precautions. After contact with the skin or eyes, the substance should also be washed off with running water. The duration of washing is at least 10 minutes.
What are acid fluxes used for?
Acid provides the best environment for solder contact with parts over the largest possible area:
Figure 2. The structure of a salt battery.
- cleaning the treated surfaces from oxides and contaminants;
- protecting them from the resumption of the oxidation process;
- significantly reducing the surface tension of the solder, which allows it to flow more freely.
The result of this is a more reliable connection of the parts being soldered.
Different metals require the use of different soldering acids, but you should immediately understand that acidic fluxes should not be used when assembling circuit boards, because they are an aggressive environment that can have a destructive effect on all their components. In addition, acids are excellent electrical conductors, able to create additional (and unwanted) passage channels for current. You should not rely on neutralization of the acidic environment after soldering.