Concept of temperature scale
Some non-metallic objects also have similar properties. The most common is water. A temperature scale was developed regarding the properties of the liquid that occupies a dominant position on Earth. The reference points are the temperature of changes in the aggregative states of water:
- Transformations from liquid to solid and vice versa are taken to be zero degrees.
- Boiling (vapor formation inside a liquid) at normal atmospheric pressure (760 mm Hg) is taken to be 100 ⁰C.
Attention! In addition to the Celsius scale, in practice temperature is measured in degrees Fahrenheit and on the absolute Kelvin scale. But when studying the properties of metal objects, other scales are used quite rarely.
Metal crystal lattices
In its ideal form, it is generally accepted that metals have a cubic lattice (real substances may have flaws). There are equal distances between molecules horizontally and vertically.
A solid substance is characterized by constancy:
- shapes, the object retains linear dimensions in different conditions;
- volume, the object does not change the amount of substance it occupies;
- mass, the amount of a substance expressed in grams (kilograms, tons);
- density, unit volume contains constant mass.
When transitioning into a liquid state, having reached a certain temperature, the crystal lattices are destroyed. Now we can’t talk about constancy of form. The liquid will take the form in which it is poured.
When evaporation occurs, only the mass of the substance remains constant. Gas will take up the entire volume that will be provided to it. Here we cannot say that density is a constant value.
When liquids combine, the following options are possible:
- Liquids completely dissolve in one another, as do water and alcohol. The concentration of substances will be the same throughout the entire volume.
- Liquids are stratified by density, the connection occurs only at the interface. It is only temporarily possible to obtain a mechanical mixture. Mix liquids with different properties. An example is oil and water.
Metals form alloys in the liquid state. To obtain an alloy, each of the components must be in a liquid state. With alloys, phenomena of complete dissolution of one in another are possible. Options cannot be excluded when the alloy will be obtained only as a result of intensive mixing. In this case, the quality of the alloy is not guaranteed, so they try not to mix components that do not allow obtaining stable alloys.
The resulting substances, soluble in each other, when solidified, form crystal lattices of a new type. Define:
- Heliocentered crystal lattices are also called body-centered. In the middle there is a molecule of one substance, and four more molecules of another are located around it. It is customary to call such lattices loose, since the bonds between metal molecules in them are weaker.
- Face-centered crystal lattices form compounds in which the component molecules are located on the faces. Metallurgists call such crystalline alloys dense. In reality, the density of the alloy can be higher than that of each of the components included in the composition (alchemists of the Middle Ages were looking for options for alloys in which the density would correspond to the density of gold).
Interaction with other substances
Each nonmetal has specific properties characteristic only of it, which are discussed in detail in the study of inorganic chemistry. Common properties are the ability to react with metals, hydrogen and oxygen.
When interacting with metals, most nonmetals act as oxidizing agents and exhibit a negative oxidation state in the resulting binary compounds:
- When active metals react with hydrogen, solid non-volatile hydrides are formed. Nonmetals such as silicon, phosphorus and boron are generally incapable of combining with hydrogen.
- Reactions of metals and nonmetals with chlorine form chlorides. Many metals (for example, iron) begin to burn when reacting with chlorine, forming compounds belonging to the class of salts. In this case, the chloride of a non-metal will never be a salt.
- Interaction with oxygen produces oxides, which are divided into peroxides and superoxides.
- Bonding with sulfur forms sulfides. In this case, to obtain the formula of aluminum sulfide (Al2S3), the substances need to be heated, but for sodium sulfide (Na2S), simple mechanical stirring is sufficient.
- Non-metals do not interact with water and acids.
Nonmetals can react with each other, with the more electronegative element acting as an oxidizing agent, and the less negative element becoming a reducing agent.
Melting point of metals
Different substances have different melting points. It is customary to divide metals into:
- Low-melting - it is enough to heat them to 600 ⁰C to obtain the substance in liquid form.
- Medium-melting metals melt in the temperature range 600…1600 ⁰С.
- Refractory are metals that can melt at temperatures above 1600 ⁰C.
The table shows low-melting metals in ascending order. Here you can see that the most unusual metal is mercury (Hg). Under normal conditions it is in a liquid state. This metal has the lowest melting point.
Table 1, melting and boiling points of fusible metals:
Table 2, melting and boiling points of medium-melting metals:
Table 3, melting and boiling points of refractory metals:
Various devices are used to carry out the smelting process. For example, blast furnaces are used to smelt iron. For melting non-ferrous metals, internal heating is carried out using high-frequency currents.
Molds made of non-metallic materials contain non-ferrous metals in a solid state. An alternating microwave magnetic field is created around them. As a result, the crystal lattices begin to loosen. The molecules of the substance begin to move, which causes heating within the entire mass.
If it is necessary to melt a small amount of low-melting metals, muffle furnaces are used. In them, the temperature rises to 1000...1200 ⁰С, which is enough for melting non-ferrous metals.
Ferrous metals are melted in convectors, open hearths and induction furnaces. The process involves the addition of alloying components that improve the quality of the metal.
It is most difficult to work with refractory metals. The problem is that you need to use materials that have a temperature higher than the melting point of the metal itself. The aircraft industry is currently considering the use of Titanium (Ti) as a structural material. At high flight speeds in the atmosphere, the skin heats up. Therefore, a replacement for aluminum and its alloys (AL) is needed.
The maximum melting point of this rather light metal attracts designers. Therefore, technologists are developing technological processes and equipment to produce parts from titanium and its alloys.
Halogens
The elements located in the main subgroup of the seventh group of the periodic table are chemically the most active nonmetals. Their atoms have the same number of electrons -7 in the last energy level, which explains the similarity of their chemical characteristics.
The physical properties of simple substances – non-metals – are different. Thus, fluorine and chlorine are in the gaseous phase, bromine is the liquid, and iodine is in the solid state. The activity of halogens in a group weakens with increasing charge of the atomic nucleus; fluorine is the most reactive among the halogens. In terms of reactivity, it is only surpassed by oxygen, which is part of the chalcogen group. The strength of hydrogen compounds of halogens, aqueous solutions of which are acids, increases from fluorine to iodine, and the solubility of poorly soluble salts decreases. The special position of fluorine among the halogens also concerns its ability to react with water. Halogen can decompose water to form various products: its own oxide F2O, ozone, oxygen and hydrogen peroxide.
Metal alloys
To design products from alloys, their properties are first studied. To study, the metals being studied are melted in small containers in different ratios to each other. Based on the results, graphs are built.
The lower axis represents the concentration of component A with component B. The vertical axis is temperature. Here the values of the maximum temperature are noted when all the metal is in a molten state.
When cooled, one of the components begins to form crystals. In a liquid state, eutectic is an ideal compound of metals in an alloy.
Metallurgists identify a special ratio of components at which the melting point is minimal. When making alloys, they try to select the amount of substances used in order to obtain a eutectoid alloy. Its mechanical properties are the best possible. Crystal lattices form ideal face-centered positions of atoms.
The crystallization process is studied by studying the hardening of samples upon cooling. They build special graphs where they observe how the cooling rate changes. Ready-made diagrams are available for different alloys. By marking the start and end points of crystallization, the composition of the alloy is determined.
Place of nonmetals in the system of chemical elements
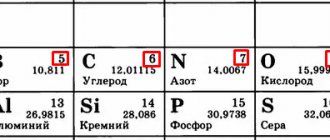
A change in the properties of atoms of non-metallic elements occurs with an increase in the atomic number. During the period, due to the increase in the charge of the nucleus, the atom contracts and its radius decreases. The oxidizing ability also increases, and the reducing properties of the elements weaken. The physical properties of nonmetals, as well as the features of their interaction with other substances, depend on the structure of their external energy level. The ability of atoms to attract foreign electrons into their sphere of influence also depends on it. For example, in the second period from boron to fluorine, the electronegativity of nonmetals increases. The most active among all non-metallic elements is fluorine. In its compounds, it holds foreign electrons the strongest, maintaining a -1 charge.
Wood's alloy
In 1860, American dental technician Barnabas Wood was looking for optimal ratios of components to produce teeth for clients at minimum melting temperatures. He found an alloy that has a melting point of only 60.2...68.5 ⁰С. Even in hot water, metal melts easily. It includes:
- tin - 12.5...12.7%;
- lead - 24.5...25.0%;
- bismuth - 49.5...50.3%;
- cadmium - 12.5...12.7%.
The alloy is interesting for its low temperature, but has never found practical application. Attention! Cadmium and lead are heavy metals and contact with them is not recommended. Many people can experience poisoning from contact with cadmium.
Soldering alloys
In practice, many people experience melting when soldering parts. If the surfaces of the materials to be joined are cleaned of contaminants and oxides, then they can be easily soldered with solders. It is customary to divide solders into hard and soft. Soft ones are most widespread:
- POS-15 - 278...282 °C;
- POS-25 - 258...262 °C;
- POS-33 - 245...249 °C;
- POS-40 - 236...241 °C;
- POS-61 - 181...185 °C;
- POS-90 - 217...222 °C.
They are produced for enterprises manufacturing various radio equipment.
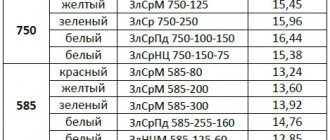
Brazing alloys based on zinc, copper, silver and bismuth have a higher melting point:
- PSr-10 - 825...835 °C;
- PSr-12 - 780...790 °C;
- PSr-25 - 760...770 °C;
- PSr-45 - 715...721 °C;
- PSr-65 - 738...743 °C;
- PSr-70 - 778...783 °C;
- PMC-36 - 823...828 °C;
- PMC-42 - 830...837 °C;
- PMC-51 - 867...884 °C.
The use of hard solders allows you to obtain strong connections.
Attention! Wed means that silver is used in the solder. Such alloys have minimal electrical resistance.
Melting point of non-metals
Non-metallic materials can be presented in solid and liquid form. Inorganic substances are presented in table. 4.
Table 4, melting point of inorganic non-metals:
In practice, organic materials are of greatest interest to users: polyethylene, polypropylene, wax, paraffin and others. The melting points of some substances are shown in table. 5.
Table 5, melting temperature of polymer materials:
Attention! The glass transition temperature refers to the state at which a material becomes brittle.
Video: melting point of known metals.
Oxygen and its features
The element is the most abundant on Earth. Its content in the soil is more than 47%, and the mass of gas in the air is 23.15%. The general physical properties of non-metals, such as nitrogen, oxygen, hydrogen, which are in the gaseous state, are determined by the structure of their molecules.
They all consist of two atoms connected by covalent nonpolar bonds. In the oxygen atom, at the last energy level there are two free p-electrons. Therefore, the oxidation state of the element is usually -2, and in compounds with fluorine (for example, OF2) +2. Oxygen is poorly soluble in water; at a temperature of -183 ⁰C it turns into an easily mobile blue liquid that can be attracted by a magnet. The element is represented by two simple substances: oxygen O2 and ozone O3. The characteristic smell of ozone can be felt in the air after a thunderstorm. The substance is extremely aggressive, decomposes organic materials and oxidizes even passive metals such as platinum or gold. Most complex substances - oxides, salts, bases and acids - contain oxygen atoms in their molecules.