- home
- Plasmacenter company services
- Coating
- Tungsten Carbide Coating
/
/
/
Tungsten carbide is a widely known alloy with high hardness and increased wear-resistant properties.
The hardness of tungsten carbide (WC) is more than 60 HRC. Elastic modulus – 69 GPa. WC coatings have high strength properties, but are fragile and difficult to process. We offer tungsten carbide coating using the following technologies:
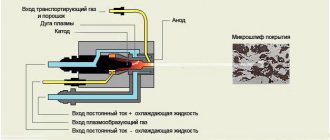
- Plasma spraying,
- Gas flame spraying,
- Detonation spraying,
- High speed spraying,
- Electric spark alloying,
- Plasma surfacing.
Each technology has its own characteristics
The tungsten carbide coating is applied using the electric spark alloying method with a thickness of 10-100 microns. In this case, an electrode made of tungsten carbide with the addition of cobalt is used. The coating obtained by detonation and high-speed spraying has minimal porosity. As a rule, pure tungsten carbide is not applied by sputtering methods. Combined materials are used - a softer and more plastic material, with the addition of solid WC particles. Such coatings are considered more wear-resistant. The thickness of the tungsten carbide coating using sputtering technologies is 100-300 microns.
Electrodeposition of tungsten from molten salts.
Tungsten cannot be isolated in its pure form from aqueous solutions, since it is more electronegative than hydrogen. Aqueous electrolytes can be used to deposit tungsten alloys with nickel, iron and cobalt.
There are works in the literature on the production and electrorefining of tungsten from oxide and halide-oxide melts, but these works mainly relate to the production of tungsten powders.
Only a small number of studies contain data on the electrodeposition of solid tungsten coatings, which are obtained almost exclusively either from pure oxide melts or with halide additions.
One of the first works on the electrolysis of oxide melts was the work of Van Lympt, carried out in 1925. Alkali metal tungstates and their mixtures were studied. For tungstening, a weakly acidic electrolyte with a tungsten trioxide concentration of up to 5 mol.% is recommended. Electrolysis is carried out at a temperature of 900-1050o C in the range of cathode current densities from 20 to 80 A/dm2. Tungsten coatings with a thickness of 20 to 100 microns were obtained on copper and nickel substrates. Thicker sediments are obtained by repeated precipitation. A.N. Baraboshkin and his co-workers carried out systematic studies of cathodic precipitation products from molten tungsten systems depending on deposition conditions; temperature, electrolyte composition, cathode current plane, which made it possible to distinguish between the areas of deposition of tungsten bronzes and metallic tungsten. The region of tungsten release is shifted towards high temperatures and concentrations of tungsten trioxide up to 20 mol.%.
Continuous tungsten coatings up to 150 microns thick can be obtained on copper, nickel, graphite, molybdenum and tungsten by electrolysis of a polytungsten bath of the composition Na2WO4 - 20 mol.% WO3 in the temperature range 815-900o C and cathode current densities 0.01-0.1 A /cm2. The sediments have a coarse-crystalline structure, as a result of which even at thicknesses of 150-200 microns they are very rough. It was found that epitaxy has a significant effect. The size of the grain in the sediment is determined by the size of the grains in the substrate. Metal microhardness 380-480 kg/mm2. The coatings had an axial <111> texture, usually not very strong. The faceting of the growing surface of the tungsten deposit is formed by smooth planes of the {112} family. The grains had a twin structure.
To refine the grains in the sediment and thereby increase the thickness of the continuous coating, carbon dioxide was introduced into the atmosphere above the melt. With an increase in the partial pressure of carbon dioxide, the sediments become finely crystalline, but the columnar structure is retained. An increase in microhardness to 500-560 kg/mm2 and an increase in the carbon content in the sediment to 0.1-0.3 wt.% are observed.
The same authors tried to reduce the grain size in the deposit by applying cathodic current pulses both at the beginning and during electrolysis. Initial current pulses of up to 20 A/cm2 crush the grain in the sediment.
The greater the pulse amplitude, the stronger this effect. Pulses applied during the growth of a continuous tungsten layer do not disturb the monocrystalline nature of the sediment grains and only cause an increase in the defectiveness of the layer.
Electrodeposition of tungsten from polytungsten melts was carried out in air; crucibles made of alundum or quartz served as containers for the melt. These materials interact with the melt, which leads to contamination of the tungsten deposit with aluminum or silicon. The aluminum and silicon contents in some sediments were 0.1 and 0.3 wt.%, respectively.
The disadvantage of a purely tungstate bath is the high concentration of tungsten in the melt. Either oxide or halide melts are used as diluents.
Davis and Gentry used a tungstate-metaborate bath to produce solid tungsten deposits. Electrolysis was carried out in a nitrogen atmosphere. Continuous tungsten deposits up to 500 microns thick were obtained on nickel and molybdenum substrates at a temperature of 900 ° C and cathode current densities of 0.010-0.030 A/cm2. The current efficiency was 85-100%. Tungsten microhardness is 425 kg/mm2. The sediments had a weak texture with an axis of <100>. McCawley and his co-authors improved this bathtub. Replacing the nitrogen atmosphere with argon and more thorough dehydration of the melt made it possible to obtain smooth and well-adhered deposits up to 650 microns thick. Electrolysis was carried out with cathodes made of nickel, molybdenum and stainless steel. The anode is pure tungsten. The cathode rotated at a speed of 150-200 rpm. The cathode current density varied within the range of 0.04-0.06 A/cm2, the temperature was 900 ° C. A decrease in temperature causes the deposition of tungsten in the form of a dark, loose powder.
In an electrolyte of the composition (wt.%): CaCl2 - 87, CaWO4 - IO, CaO-3, tungsten layers with a thickness of 50 - 60 µm with a cathodic current output of a solid deposit of 50-70%. As the sediment thickens, the tungsten grains become larger, which ultimately leads to the progressive growth of individual protrusions and their transformation into dendrites. The addition of calcium oxide to the melt refines the grains in the cathode deposit and makes it possible to obtain pore-free coatings with a thickness of 150-170 microns. An increase in the cathode current density from 0.3 to 1 A/cm2 causes a sharp grain refinement and an increase in roughness, which leads to a limitation of the thickness of the continuous deposit to 10-15 microns. The coatings had texture. The dependence of the degree of texture perfection on the cathode current density, tungstate and calcium oxide concentrations is extreme. The maximum on these curves corresponds to a cathodic current density of 0.1 A/cm2, a tungstate concentration of 10 wt.% and calcium oxide of 0.5 wt.%. The experiments were carried out in an alundum crucible in an air atmosphere.
In the chloride-oxide melt (mol.%): NaCl-KCl (I:I) - 85-95, alkali metal tungstate 2-10, alkali metal metaphosphate 0.25-2, alkali metal pyrophosphate I-3 at a temperature of 7000C and At current densities of 0.02-0.05 A/cm2, continuous tungsten coatings up to 150 microns thick were obtained.
Compact layers of tungsten with a thickness of 5-6 microns were deposited in a melt of the following composition (wt.%) during a single electrolysis cycle at a temperature of 850-9000C and a cathode current density of 0.6-0.8 A/cm2: NaCl-79, Na2WO4-20, Na2CO3 - 1. The authors were unable to increase the thickness of the coating when using pulsating and superimposed alternating current in various modes.
The electrodeposition of tungsten from an oxide-halide melt (wt.%) was studied: NaCl - 60, Na3WO3 - 40. Electrolysis was carried out at current densities of 0.01-0.1 A/cm2 and temperatures of 840-920°C. At 920°C and current densities of 0.01-0.02 A/cm2, compact fine-crystalline tungsten coatings are deposited. With increasing current density, the deposits become coarse-crystalline, and the continuity of the coating is disrupted due to the intensive development of dendrites. Thick continuous layers are obtained by repeating the process multiple times or with periodic anodic etching in the same melt after passing 0.1 A-hour/cm2. The microhardness of tungsten coatings is 420-450 kg/mm2.
There are reports of the use of halide electrolytes for refining, obtaining tungsten powders and coatings.
Mellors and Senderof proposed to obtain thick (up to several mm) tungsten coatings using a fluoride melt of the following composition (wt.%): 70-90% eutectic KF - NaF and 10-30% tungsten fluoride. Electrodeposition is carried out in an inert atmosphere, at a temperature of 700-900o C and a cathodic current density of 0.002-0.2 A/cm2. The structure of sediments is columnar. The microhardness of sediments was 400-450 kg/mm2. Impurities of chlorine, bromine and oxygen anions are allowed in very small quantities, as they cause the formation of porous deposits.
The modes of deposition of continuous layers of tungsten from fluoride melts were studied in detail. It is noted that at high concentrations of tungsten ions in the melt (150 wt.% and above), continuous deposits can be obtained at high temperatures - 900 ° C and above. At tungsten ion concentrations of 1-5 wt.%, continuous layers can be obtained at 700-800o C - the lower the temperature, the lower the current density (0.07-0.1 and 0.01 A/cm2 at 800 and 700o C, respectively) . The sediments had a well-defined columnar structure and, in most cases, a <111> texture. The grains in the sediments had a twin structure. The microhardness of sediments was 440-500 kg/mm2. In long-term experiments, the normal course of electrolysis is disrupted over time: the cathode current efficiency drops sharply to 10-20%.
Suchkov and co-authors proposed using a chloride-fluoride melt of the following composition (wt.%) to obtain fine tungsten powder: KF - 38-42, KCI - 38-42, WCl6 - 16-24. For electrorefining, a melt of composition (wt.%) is used: 60 KCI-30 NaF-10 WCl6. Electrolysis was carried out in the temperature range 700-800°C at a cathodic current density of 0.6 A/cm2. The cathode current efficiency was 74-84%.
Ervin and Heltz proposed using a melt of tungsten chloride and alkali or alkaline earth metal chlorides to produce pure tungsten. Current density 0.025 A/cm2, temperature 900o C. Tungsten is deposited in the form of a sponge.
The electrolysis of chloride electrolytes is described: KCl-NaCl-WCl6, LiCl-KCl-WCl6. However, the authors were unable to obtain continuous tungsten layers and these melts were considered unpromising due to their instability. The cathode deposits had the form of a fine black powder, and the current efficiency did not exceed 15%.
Tungsten powders were obtained in a melt of KCl-NaCl (1:1) + 4.8 wt.% WCl4 at temperatures of 680-900 ° C and cathode current densities of 0.2-4 A/cm2. An increase in temperature promotes the formation of coarse-crystalline precipitation. An increase in cathodic current density acts in the same direction. In the case of a short electrolysis time (10 min.), the maximum cathode current efficiency is 57%; with increasing deposition time, the current efficiency is about 26%. Electrolysis was carried out in a quartz electrolyzer in an atmosphere of purified argon.
In the only work on the electrodeposition of tungsten from chloride melts, continuous deposits with a thickness of I00 μm were obtained. Deposition was carried out in a melt of CaCl - Ca2WCl6 (4-I0 wt.% W) at temperatures of 750-800 ° C and cathodic current densities of 0.03-0.05 A/cm2. The coatings were with high microhardness - 600 kg/mm2 and non-oriented. Electrolysis was carried out in a quartz electrolyzer in an atmosphere of purified inert gas. The melt was placed in a glassy carbon crucible. It is noted that the tungsten-containing melt interacts with the quartz wall of the electrolyzer.
One of the important tasks in the development of tungsten electrodeposition processes is the choice of electrolyte, which ensures the production of continuous, non-porous coatings up to several millimeters thick with a certain structure and orientation, a high degree of purity and good mechanical properties at a high deposition rate.
Continuous tungsten layers can be deposited from three types of melts: oxide, halide-oxide and halide. From the given literature data, we can draw a conclusion about the advantages, disadvantages and the possibility of using a particular melt.
Pure oxide and halide-oxide melts do not require a protective atmosphere; they dissolve metal oxides well, which makes it possible to obtain tungsten deposits with a columnar structure on various substrates of graphite, copper, nickel and molybdenum.
However, these melts have a number of disadvantages: • The melts are quite aggressive, which makes it difficult to choose a material for the container. The instability of the container in the air atmosphere sometimes makes it necessary to create an inert atmosphere in the electrolyzer. • The maximum thickness of continuous coatings is 50-200 microns. Thicker layers are obtained only by using additional techniques to grind the grain in the sediment, which complicates the production of coatings and often worsens its properties. • Low deposition rate because the equilibrium valency of tungsten ions due to the formation of strong complexes with oxygen is higher and equal to six, and high-quality coatings are obtained only at low current densities of 0.01-0.1 A/cm2.
Despite these disadvantages, oxide and halide-oxide electrolytes can be used to obtain continuous tungsten coatings of small thickness on various metal substrates.
The use of a fluoride bath limits the toxicity, aggressiveness, and poor solubility of fluoride salts in water. The disadvantage of this melt is the use of potassium fluoride, a highly hygroscopic compound, as a component of the melt. Insufficient dehydration leads to the deposition of porous layers.
Most tungsten deposits obtained by electrolysis of oxide, halide-oxide and fluoride melts had an axial texture <111>. The grains in the sediment are twins. The faceting of the growing surface of the sediment is formed by planes of the {112} family. The perfection of the texture is determined by the electrodeposition conditions: melt composition, temperature, cathode current density. It is known that chloride melts are successfully used for deposition of coatings from such refractory metals as molybdenum, rhenium, niobium, vanadium. Therefore, the precipitation of tungsten from chloride melts is of great interest. Compared to other electrolytes, chloride melts have a number of advantages: relatively low melting point, high decomposition potential, good solubility in water, non-toxic, non-aggressive. The strength of the complexes and the low volatility of fluorides compared to chlorides determine their advantages.
Therefore, the electrodeposition of tungsten from a chloride-fluoride bath, which combines the advantages of chlorides and fluorides, is also of interest.
As can be seen from the above literature data, there are many different melts for obtaining continuous layers of tungsten, but none of them, except for the fluoride electrolyte, made it possible to deposit thick deposits. They were not obtained from chloride melts either. This, apparently, is not a consequence of the specificity of chloride electrolytes as media for electrodeposition, but is due to the fact that the studies did not take into account the characteristics of both the chloride melt and metal tungsten.
The peculiarities of chloride melts include their sensitivity to the purity of the experiment and especially to oxygen-containing impurities. Tungsten halides have a high affinity for oxygen, as a result of which oxygen-containing materials cannot be used as containers. In tungsten, the solubility of interstitial impurities (oxygen and carbon) is insignificant, and it decreases with decreasing temperature.
Photo
"Plasmacenter" offers
- services for restoration of parts, coating, vacuum sputtering, microplasma sputtering, electric spark alloying, plasma processing, coating certification, titanium nitride sputtering, shaft repair, corrosion coating, protective coating, hardening of parts;
- supply of equipment for the processes of finishing plasma hardening, welding, soldering, surfacing, spraying (for example, gas-thermal, gas-flame, microplasma, high-speed and detonation spraying), electric spark alloying, control devices, powder dispensers, plasma torches and other equipment;
- supply of consumables such as welding wire, electrodes, welding rods, spraying powders, surfacing powders, additive manufacturing powders, surfacing wire and other materials for welding, surfacing, spraying, additive manufacturing and hardening processes;
- conducting R&D in the field of surface engineering, coating tribology, plasma processing methods, selection of optimal coatings and methods of their application;
- training, consulting in the field of surfacing, sputtering, hardening, modification, hardening.
Contact us by phone: +7 (812) 679-46-74, +7 (921) 973-46-74, or write to us by email
Our managers will tell you in detail about our available technologies for coating, hardening, restoration, imparting surface properties, as well as the cost of the company’s services.
Tungsten carbide coating as an alternative to galvanic chrome plating.
For more than 70 years, chrome coatings have remained essential for protecting aviation components, industrial and consumer products from wear, impact and corrosion. However, in recent years, the disadvantages of chrome-plated surfaces have forced the engineering community to look for cheaper and more effective ways to protect surfaces in both the military and civil aviation sectors, as well as in industry. The best alternative to chrome plating today is considered to be high-velocity flame spraying (HVOF)
of tungsten carbide. Evaluation testing and increasing number of successful industrial applications of tungsten carbide HVOF coatings for various aircraft engine and airframe components are proving their superiority. These coatings are used on aircraft landing gear, hydraulic cylinders, jet engine bearings and bearing housings, turbine shafts, and even on items such as helicopter drive chains and propeller assemblies. Providing better protection against wear, impact and fatigue, and better or equivalent protection against corrosion, these coatings are gradually replacing chrome plating.
In addition to the benefits of HVOF tungsten carbide coatings in harsh environments, these coatings are much easier to apply compared to traditional chromium electrolytic baths. Indeed, a large number of published technological evaluations (both military and civilian) demonstrate the viability of HVOF coatings as a replacement for chrome plating. To date, a large number of laboratory and pilot tests, commercial operation have demonstrated the advantages of HVOF coatings in protection against wear, corrosion and overheating; labor-intensive application; life cycle duration; economic efficiency.