CARBON (lat. Сarboneum), C, chemical. element of group IV of the short form (group 14 of the long form) periodic. systems; at. n. 6, at. m. 12.0107. Natural uranium consists of two stable isotopes: 12C (98.89%) and 13C (1.11%). The atmosphere contains the radioisotope 14C (T1/2 5730 years, β-emitter), which is formed in the upper layers of the atmosphere when 14N nuclei are irradiated by cosmic neutrons. radiation. According to the content of the 14C isotope in plants. and animal remains are determined by their age (radiocarbon dating method). Radioisotopes with mass numbers 8–22 have been artificially obtained.
Historical reference.
Graphite, diamond and amorphous carbon have been known since antiquity. It has long been known that graphite can be used to mark other materials, and the name “graphite” itself, which comes from the Greek word meaning “to write”, was proposed by A. Werner in 1789. However, the history of graphite is complicated; substances with similar external physical properties were often mistaken for it , such as molybdenite (molybdenum sulfide), at one time considered graphite. Other names for graphite include “black lead,” “iron carbide,” and “silver lead.” In 1779, K. Scheele established that graphite can be oxidized with air to form carbon dioxide.
Diamonds first found use in India, and in Brazil gems became commercially important in 1725; deposits in South Africa were discovered in 1867. In the 20th century. The main diamond producers are South Africa, Zaire, Botswana, Namibia, Angola, Sierra Leone, Tanzania and Russia. Man-made diamonds, the technology of which was created in 1970, are produced for industrial purposes.
Allotropy.
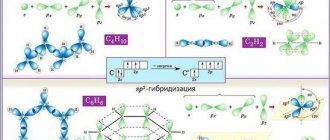
If the structural units of a substance (atoms for monoatomic elements or molecules for polyatomic elements and compounds) are able to combine with each other in more than one crystalline form, this phenomenon is called allotropy. Carbon has three allotropic modifications: diamond, graphite and fullerene. In diamond, each carbon atom has 4 tetrahedrally located neighbors, forming a cubic structure (Fig. 1a). This structure corresponds to the maximum covalency of the bond, and all 4 electrons of each carbon atom form high-strength C–C bonds, i.e. There are no conduction electrons in the structure. Therefore, diamond is characterized by its lack of conductivity, low thermal conductivity, and high hardness; it is the hardest known substance (Fig. 2). Breaking the C–C bond (bond length 1.54 Å, hence covalent radius 1.54/2 = 0.77 Å) in a tetrahedral structure requires large amounts of energy, so diamond, along with exceptional hardness, is characterized by a high melting point (3550 °C).
Another allotropic form of carbon is graphite, which has very different properties from diamond. Graphite is a soft black substance made of easily exfoliated crystals, characterized by good electrical conductivity (electrical resistance 0.0014 Ohm cm). Therefore, graphite is used in arc lamps and furnaces (Fig. 3), in which it is necessary to create high temperatures. High purity graphite is used in nuclear reactors as a neutron moderator. Its melting point at elevated pressure is 3527° C. At normal pressure, graphite sublimates (transforms from solid to gas) at 3780° C.
The structure of graphite (Fig. 1b) is a system of condensed hexagonal rings with a bond length of 1.42 Å (much shorter than in diamond), but each carbon atom has three (not four, as in diamond) covalent bonds with three neighbors, and the fourth bond (3.4 Å) is too long for a covalent bond and weakly connects parallel graphite layers to each other. It is the fourth electron of carbon that determines the thermal and electrical conductivity of graphite - this longer and less strong bond forms the less compactness of graphite, which is reflected in its lower hardness compared to diamond (graphite density 2.26 g/cm3, diamond - 3.51 g/cm3 cm3). For the same reason, graphite is slippery to the touch and easily separates flakes of the substance, which is why it is used to make lubricant and pencil leads. The lead-like sheen of the lead is mainly due to the presence of graphite.
Carbon fibers have high strength and can be used to make rayon or other high carbon yarns.
At high pressure and temperature in the presence of a catalyst such as iron, graphite can transform into diamond. This process is implemented for the industrial production of artificial diamonds. Diamond crystals grow on the surface of the catalyst. The graphite-diamond equilibrium exists at 15,000 atm and 300 K or at 4000 atm and 1500 K. Artificial diamonds can also be obtained from hydrocarbons.
Amorphous forms of carbon that do not form crystals include charcoal, obtained by heating wood without access to air, lamp and gas soot, formed during low-temperature combustion of hydrocarbons with a lack of air and condensing on a cold surface, bone char - an admixture to calcium phosphate in the process of bone destruction fabrics, as well as coal (a natural substance with impurities) and coke, a dry residue obtained from the coking of fuels by the method of dry distillation of coal or petroleum residues (bituminous coals), i.e. heating without air access. Coke is used for smelting cast iron and in ferrous and non-ferrous metallurgy. Coking also produces gaseous products - coke oven gas (H2, CH4, CO, etc.) and chemical products, which are raw materials for the production of gasoline, paints, fertilizers, medicines, plastics, etc. A diagram of the main apparatus for coke production - a coke oven - is shown in Fig. 3.
Various types of coal and soot have a developed surface and are therefore used as adsorbents for purifying gas and liquids, and also as catalysts. To obtain various forms of carbon, special methods of chemical technology are used. Artificial graphite is produced by calcining anthracite or petroleum coke between carbon electrodes at 2260°C (Acheson process) and is used in the production of lubricants and electrodes, in particular for the electrolytic production of metals.
Story
Carbon in the form of charcoal was used in ancient times to smelt metals.
Allotropic modifications of carbon—diamond and graphite—have long been known. At the turn of the XVII-XVIII centuries. The phlogiston theory arose, put forward by Johann Becher and Georg Stahl. This theory recognized the presence in each combustible body of a special elementary substance - a weightless fluid - phlogiston, which evaporates during the combustion process. Since when a large amount of coal is burned, only a little ash remains, phlogistics believed that coal was almost pure phlogiston. This is what explained, in particular, the “phlogisticating” effect of coal—its ability to restore metals from “limes” and ores. The later phlogistics, Reaumur, Bergman and others, had already begun to understand that coal was an elemental substance. However, “clean coal” was first recognized as such by Antoine Lavoisier, who studied the process of combustion of coal and other substances in air and oxygen. In the book “Method of Chemical Nomenclature” (1787) by Guiton de Morveau, Lavoisier, Berthollet and Fourcroix, the name “carbon” (carbone) appeared instead of the French “pure coal” (charbone pur). Under the same name, carbon appears in the “Table of Simple Bodies” in Lavoisier’s “Elementary Textbook of Chemistry.”
In 1791, the English chemist Tennant was the first to obtain free carbon; he passed phosphorus vapor over calcined chalk, resulting in the formation of calcium phosphate and carbon. It has been known for a long time that diamond burns without leaving a residue when heated strongly. Back in 1751, the German Emperor Franz I agreed to provide diamond and ruby for combustion experiments, after which these experiments even became fashionable. It turned out that only diamond burns, and ruby (aluminum oxide with an admixture of chromium) can withstand prolonged heating at the focus of the ignition lens without damage. Lavoisier conducted a new experiment on burning diamond using a large incendiary machine and came to the conclusion that diamond is crystalline carbon. The second allotrope of carbon - graphite - in the alchemical period was considered a modified lead luster and was called plumbago; It was only in 1740 that Pott discovered the absence of any lead impurity in graphite. Scheele studied graphite (1779) and, being a phlogistician, considered it a special kind of sulfur body, a special mineral coal containing bound “aerial acid” (CO2) and a large amount of phlogiston.
Twenty years later, Guiton de Morveau turned the diamond into graphite and then into carbonic acid by careful heating.
origin of name
In the 17th-19th centuries, the term “carbon solution” was sometimes used in Russian chemical and specialized literature (Schlatter, 1763; Scherer, 1807; Severgin, 1815); Since 1824, Solovyov introduced the name “carbon”. Carbon compounds have the carbo(n)
- from lat.
carbō (gen. carbōnis
) “coal”.
Structure of the carbon atom.
The nucleus of the most stable carbon isotope, mass 12 (98.9% abundance), has 6 protons and 6 neutrons (12 nucleons), arranged in three quartets, each containing 2 protons and two neutrons, similar to the helium nucleus. Another stable isotope of carbon is 13C (about 1.1%), and in trace amounts there exists in nature an unstable isotope 14C with a half-life of 5730 years, which has b-radiation. All three isotopes participate in the normal carbon cycle of living matter in the form of CO2. After the death of a living organism, carbon consumption stops and C-containing objects can be dated by measuring the level of 14C radioactivity. The decrease in 14CO2 b-radiation is proportional to the time that has passed since death. In 1960, W. Libby was awarded the Nobel Prize for his research with radioactive carbon. See also DATING BY RADIOACTIVITY.
In the ground state, 6 electrons of carbon form the electronic configuration 1s22s22px12py12pz0. Four electrons of the second level are valence, which corresponds to the position of carbon in group IVA of the periodic table (see PERIODIC SYSTEM OF ELEMENTS). Since large energy is required to remove an electron from an atom in the gas phase (approx. 1070 kJ/mol), carbon does not form ionic bonds with other elements, since this would require the removal of an electron to form a positive ion. Having an electronegativity of 2.5, carbon does not exhibit strong electron affinity and, accordingly, is not an active electron acceptor. Therefore, it is not prone to form a particle with a negative charge. But some carbon compounds exist with a partially ionic nature of the bond, for example carbides. In compounds, carbon exhibits an oxidation state of 4. In order for four electrons to participate in the formation of bonds, it is necessary to pair the 2s electrons and jump one of these electrons to the 2pz orbital; in this case, 4 tetrahedral bonds are formed with an angle between them of 109°. In compounds, carbon's valence electrons are only partially withdrawn from it, so carbon forms strong covalent bonds between neighboring C–C atoms using a shared electron pair. The breaking energy of such a bond is 335 kJ/mol, whereas for the Si–Si bond it is only 210 kJ/mol, so long –Si–Si– chains are unstable. The covalent nature of the bond is preserved even in compounds of highly reactive halogens with carbon, CF4 and CCl4. Carbon atoms are capable of donating more than one electron from each carbon atom to form a bond; This is how double C=C and triple CєC bonds are formed. Other elements also form bonds between their atoms, but only carbon is capable of forming long chains. Therefore, for carbon, thousands of compounds are known, called hydrocarbons, in which the carbon is bonded to hydrogen and other carbon atoms to form long chains or ring structures. See ORGANIC CHEMISTRY.
In these compounds, it is possible to replace hydrogen with other atoms, most often with oxygen, nitrogen and halogens to form a variety of organic compounds. Fluorocarbons are important among them - hydrocarbons in which hydrogen is replaced by fluorine. Such compounds are extremely inert, and they are used as plastic and lubricants (fluorocarbons, i.e. hydrocarbons in which all hydrogen atoms are replaced by fluorine atoms) and as low-temperature refrigerants (chlorofluorocarbons, or freons).
In the 1980s, US physicists discovered very interesting carbon compounds in which carbon atoms are connected into 5- or 6-gons, forming a C60 molecule in the shape of a hollow ball with the perfect symmetry of a soccer ball. Since this design forms the basis of the “geodesic dome” invented by the American architect and engineer Buckminster Fuller, the new class of compounds was called “buckminsterfullerenes” or “fullerenes” (and also more briefly “phasyballs” or “buckyballs”). Fullerenes - the third modification of pure carbon (except for diamond and graphite), consisting of 60 or 70 (or even more) atoms - were obtained by the action of laser radiation on the smallest particles of carbon. Fullerenes of more complex shapes consist of several hundred carbon atoms. The diameter of the C60 molecule is ~ 1 nm. In the center of such a molecule there is enough space to accommodate a large uranium atom. See also FULLERENES.
Being in nature
The carbon content in the earth's crust is 0.1% by mass. Free carbon is found in nature in the form of diamond and graphite. The bulk of carbon is in the form of natural carbonates (limestones and dolomites), fossil fuels - anthracite (94-97% C), brown coal (64-80% C), bituminous coal (76-95% C), oil shale (56- 78% C), oil (82-87% C), flammable natural gases (up to 99% methane), peat (53-56% C), as well as bitumen, etc. In the atmosphere and hydrosphere it is found in the form of carbon dioxide CO2, in the air there is 0.046% CO2 by mass, in the waters of rivers, seas and oceans it is ~60 times more. Carbon is included in the composition of plants and animals (~18%). The human body enters carbon through food (normally about 300 g per day). The total carbon content in the human body reaches about 21% (15 kg per 70 kg body weight). Carbon makes up 2/3 of muscle mass and 1/3 of bone mass. Excreted from the body mainly through exhaled air (carbon dioxide) and urine (urea). The carbon cycle in nature includes the biological cycle, the release of CO2 into the atmosphere during the combustion of fossil fuels, from volcanic gases, hot mineral springs, from the surface layers of ocean waters, etc. Biological The cycle is that carbon in the form of CO2 is absorbed from the troposphere by plants. Then from the biosphere it returns again to the geosphere: with plants, carbon enters the body of animals and humans, and then, when animal and plant materials rot, into the soil and in the form of CO2 into the atmosphere.
In a vapor state and in the form of compounds with nitrogen and hydrogen, carbon is found in the atmosphere of the Sun, planets, and is found in stone and iron meteorites.
Most carbon compounds, and above all hydrocarbons, have a pronounced character of covalent compounds. The strength of simple, double and triple bonds of C atoms with each other, the ability to form stable chains and cycles from C atoms determine the existence of a huge number of carbon-containing compounds studied in organic chemistry.
Chemical properties of carbon and some of its compounds.
Some physical and chemical properties of carbon are given in the article CHEMICAL ELEMENTS. The reactivity of carbon depends on its modification, temperature and dispersion. At low temperatures, all forms of carbon are quite inert, but when heated they are oxidized by atmospheric oxygen, forming oxides:
Finely dispersed carbon in excess oxygen can explode when heated or from a spark. In addition to direct oxidation, there are more modern methods for producing oxides.
Physical properties
Carbon exists in a variety of allotropes with very diverse physical properties. The variety of modifications is due to the ability of carbon to form chemical bonds of different types.
Carbon isotopes
Main article: Carbon isotopes
Natural carbon consists of two stable isotopes - 12C (98.93%) and 13C (1.07%) and one radioactive isotope 14C (β-emitter, T½ = 5730 years), concentrated in the atmosphere and upper part earth's crust. It is constantly formed in the lower layers of the stratosphere as a result of the impact of neutrons from cosmic radiation on nitrogen nuclei according to the reaction: 14N (n, p) 14C, and also, since the mid-1950s, as a man-made product of nuclear power plants and as a result of testing hydrogen bombs.
The formation and decay of 14C is the basis of the radiocarbon dating method, which is widely used in Quaternary geology and archaeology.
Carbon suboxide
C3O2 is formed by dehydration of malonic acid over P4O10:
C3O2 has an unpleasant odor and is easily hydrolyzed, again forming malonic acid.
| PHYSICAL PROPERTIES OF CARBON OXIDES | |||
| CO | CO2 | C3O2 | |
| Molecular mass | 28,010 | 44,010 | 68,030 |
| Boiling point, °C | –192 | –56,6 | –7 |
| Freezing temperature, °C | –199 | –78,5 | –111,3 |
| Density, g/l (at 0° C) | 1,250 | 1,977 | 1,114 |
| Solubility, volume/volume of water (at 0°C) | 0,03 | 1,7 | Reacts with water |
Carbon monoxide (II) CO is formed during the oxidation of any modification of carbon under conditions of lack of oxygen. The reaction is exothermic, 111.6 kJ/mol is released. Coke reacts with water at white heat temperature: C + H2O = CO + H2; the resulting gas mixture is called “water gas” and is a gaseous fuel. CO is also formed during incomplete combustion of petroleum products; it is found in noticeable quantities in automobile exhausts; it is obtained during the thermal dissociation of formic acid:
The oxidation state of carbon in CO is +2, and since carbon is more stable in the oxidation state +4, CO is easily oxidized by oxygen to CO2: CO + O2 → CO2, this reaction is highly exothermic (283 kJ/mol). CO is used in industry in a mixture with H2 and other flammable gases as a fuel or gaseous reducing agent. When heated to 500° C, CO forms C and CO2 to a noticeable extent, but at 1000° C, equilibrium is established at low concentrations of CO2. CO reacts with chlorine, forming phosgene - COCl2, reactions with other halogens proceed similarly, in the reaction with sulfur carbonyl sulfide COS is obtained, with metals (M) CO forms carbonyls of various compositions M(CO)x, which are complex compounds. Iron carbonyl is formed when blood hemoglobin reacts with CO, preventing the reaction of hemoglobin with oxygen, since iron carbonyl is a stronger compound. As a result, the function of hemoglobin as a carrier of oxygen to cells is blocked, which then die (and the brain cells are primarily affected). (Hence another name for CO – “carbon monoxide”). Already 1% (vol.) CO in the air is dangerous for humans if they are in such an atmosphere for more than 10 minutes. Some physical properties of CO are given in the table.
Carbon dioxide, or carbon monoxide (IV)CO2, is formed by the combustion of elemental carbon in excess oxygen with the release of heat (395 kJ/mol). CO2 (the trivial name is “carbon dioxide”) is also formed during the complete oxidation of CO, petroleum products, gasoline, oils and other organic compounds. When carbonates are dissolved in water, CO2 is also released as a result of hydrolysis:
This reaction is often used in laboratory practice to produce CO2. This gas can also be obtained by calcination of metal bicarbonates:
during gas-phase interaction of superheated steam with CO:
when burning hydrocarbons and their oxygen derivatives, for example:
Similarly, food products are oxidized in a living organism, releasing heat and other types of energy. In this case, oxidation occurs under mild conditions through intermediate stages, but the end products are the same - CO2 and H2O, as, for example, during the decomposition of sugars under the action of enzymes, in particular during the fermentation of glucose:
Large-scale production of carbon dioxide and metal oxides is carried out in industry by the thermal decomposition of carbonates:
CaO is used in large quantities in cement production technology. The thermal stability of carbonates and the heat consumption for their decomposition according to this scheme increase in the series CaCO33 3. Carbon dioxide is a chemically inactive compound, but in some cases it can support the combustion process, for example, magnesium burns in a CO2 environment. Materials that burn at low temperatures - wood, oil products, paper, etc. - do not burn in a CO2 environment. Therefore, and also because of its higher density than air (CO2 is 1.5 times heavier), carbon dioxide is used in fire extinguishers. In its solid state, CO2 is known as “dry ice.” At high concentrations, CO2 is dangerous for humans, as it blocks the access of oxygen (see also FIRE PREVENTION AND FIRE PROTECTION).
Application
Graphite is used in the pencil industry. It is also used as a lubricant at particularly high or low temperatures.
Diamond, due to its exceptional hardness, is an indispensable abrasive material. The grinding attachments of drills are coated with diamond. In addition, cut diamonds are used as gemstones in jewelry. Due to its rarity, high decorative qualities and a combination of historical circumstances, a diamond is invariably the most expensive gemstone. The exceptionally high thermal conductivity of diamond (up to 2000 W/m•K) makes it a promising material for semiconductor technology as substrates for processors. But the relatively high price (about $50/gram) and the difficulty of diamond processing limit its use in this area. In pharmacology and medicine, various carbon compounds are widely used - derivatives of carbonic acid and carboxylic acids, various heterocycles, polymers and other compounds. Thus, carbolene (activated carbon) is used to absorb and remove various toxins from the body; graphite (in the form of ointments) - for the treatment of skin diseases; radioactive carbon isotopes—for scientific research (radiocarbon dating).
Carbon plays a huge role in human life. Its applications are as varied as this many-sided element itself.
Carbon is the basis of all organic substances. Any living organism consists largely of carbon. Carbon is the basis of life. The source of carbon for living organisms is usually CO2 from the atmosphere or water. Through photosynthesis, it enters biological food chains in which living things devour each other or each other's remains and thereby obtain carbon to build their own bodies. The biological cycle of carbon ends either by oxidation and return to the atmosphere, or by burial in the form of coal or oil.
Carbon in the form of fossil fuels: coal and hydrocarbons (oil, natural gas) is one of the most important sources of energy for humanity.
Carbonates.
Carbonates are formed by the interaction of metal oxides with CO2, for example, Na2O + CO2 Na2CO3.
With the exception of alkali metal carbonates, the rest are practically insoluble in water, and calcium carbonate is partially soluble in carbonic acid or a solution of CO2 in water under pressure:
CaCO3 + H2O + CO2 Ca(HCO3)2
These processes occur in groundwater flowing through the limestone layer. Under conditions of low pressure and evaporation, CaCO3 precipitates from groundwater containing Ca(HCO3)2. This is how stalactites and stalagmites grow in caves. The color of these interesting geological formations is explained by the presence of impurities in the waters of iron, copper, manganese and chromium ions. Carbon dioxide reacts with metal hydroxides and their solutions to form bicarbonates, for example:
NaOH + CO2 → NaHCO3
which, when heated, decompose releasing CO2:
2NaHCO3 → Na2CO3 + H2O + CO2
Sodium carbonate, or soda, is produced in large quantities in the soda industry, mainly by the Solvay method:
Another method is to obtain soda from CO2 and NaOH
The carbonate ion CO32– has a flat structure with an O–C–O angle of 120° and a CO bond length of 1.31 Å (see also ALKALI PRODUCTION).
[edit] Education
The formation of a carbon atom requires the almost simultaneous collision of three alpha particles, that is, the nuclei of a helium atom. Such a process, known as the triple alpha process, can only occur in the interior of giant or supergiant stars[1] with high density and high temperature (about 100 thousand K). To get to Earth, carbon must first leave the parent star where it was formed (for example, as a result of a supernova explosion) and enter interstellar space[2]. Third generation stellar systems, which include the Solar System, were formed from the interstellar medium, which was enriched in elements heavier than helium.
Carbon halides.
Carbon reacts directly with halogens when heated to form tetrahalides, but the reaction rate and product yield are low. Therefore, carbon halides are obtained by other methods, for example, CCl4 is obtained by chlorination of carbon disulfide:
CS2 + 2Cl2 ® CCl4 + 2S
Tetrachloride CCl4 is a non-flammable substance, used as a solvent in dry cleaning processes, but it is not recommended to use it as a flame arrester, since at high temperatures toxic phosgene (a gaseous toxic substance) is formed. CCl4 itself is also poisonous and, if inhaled in significant quantities, can cause liver poisoning. СCl4 is also formed by the photochemical reaction between methane СH4 and Сl2; in this case, the formation of products of incomplete chlorination of methane - CHCl3, CH2Cl2 and CH3Cl - is possible. Reactions occur similarly with other halogens.
Reactions of graphite.
Graphite, as a modification of carbon, characterized by large distances between the layers of hexagonal rings, enters into unusual reactions, for example, alkali metals, halogens and some salts (FeCl3) penetrate between the layers, forming compounds such as KC8, KC16 (called interstitial compounds, inclusions or clathrates). Strong oxidizing agents such as KClO3 in an acidic environment (sulfuric or nitric acid) form substances with a large volume of the crystal lattice (up to 6 Å between layers), which is explained by the introduction of oxygen atoms and the formation of compounds on the surface of which, as a result of oxidation, carboxyl groups (–COOH) are formed – compounds such as oxidized graphite or mellitic (benzene hexacarboxylic) acid C6(COOH)6. In these compounds, the C:O ratio can vary from 6:1 to 6:2.5.
Carbides.
Carbon forms various compounds called carbides with metals, boron and silicon. The most active metals (IA–IIIA subgroups) form salt-like carbides, for example Na2C2, CaC2, Mg4C3, Al4C3. In industry, calcium carbide is obtained from coke and limestone using the following reactions:
Carbides are non-electrically conductive, almost colorless, hydrolyze to form hydrocarbons, for example
CaC2 + 2H2O = C2H2 + Ca(OH)2
The acetylene C2H2 formed by the reaction serves as the starting material in the production of many organic substances. This process is interesting because it represents a transition from raw materials of inorganic nature to the synthesis of organic compounds. Carbides that form acetylene upon hydrolysis are called acetylenides. In silicon and boron carbides (SiC and B4C), the bond between the atoms is covalent. Transition metals (elements of B-subgroups) when heated with carbon also form carbides of variable composition in cracks on the metal surface; the bond in them is close to metallic. Some carbides of this type, for example WC, W2C, TiC and SiC, are distinguished by high hardness and refractoriness, and have good electrical conductivity. For example, NbC, TaC and HfC are the most refractory substances (mp = 4000–4200° C), diniobium carbide Nb2C is a superconductor at 9.18 K, TiC and W2C are close in hardness to diamond, and the hardness of B4C (a structural analogue of diamond ) is 9.5 on the Mohs scale (see Fig. 2). Inert carbides are formed if the radius of the transition metal is 3C, Ni3C3, Fe3C.
Nitrogen derivatives of carbon.
This group includes urea NH2CONH2 - a nitrogen fertilizer used in the form of a solution. Urea is obtained from NH3 and CO2 by heating under pressure:
Cyanogen (CN)2 has many properties similar to halogens and is often called a pseudohalogen. Cyanide is obtained by mild oxidation of cyanide ion with oxygen, hydrogen peroxide or Cu2+ ion: 2CN– ® (CN)2 + 2e.
Cyanide ion, being an electron donor, easily forms complex compounds with transition metal ions. Like CO, cyanide ion is a poison, binding vital iron compounds in a living organism. Cyanide complex ions have the general formula [M(CN)x]–0.5x, where x is the coordination number of the metal (complexing agent), empirically equal to twice the oxidation state of the metal ion. Examples of such complex ions are (the structure of some ions is given below) tetracyanonickelate(II) ion [Ni(CN)4]2–, hexacyanoferrate(III) [Fe(CN)6]3–, dicyanoargentate [Ag(CN)2] –:
[edit] Literature
- Glossary of terms in chemistry // J. Opeida, O. Shvaika. Institute of Physical-Organic Chemistry and Coal Chemistry named after. L. M. Litvinenko NAS of Ukraine, Donetsk National University - Donetsk: "Weber", 2008. - 758 p. ISBN 978-966-335-206-0
- F. A. Derkach “Chemistry” L. 1968.
- Small mountain encyclopedia. In 3 volumes / Ed. V. S. Beletsky. - Donetsk: Donbass, 2004. - ISBN 966-7804-14-3.
- Saranchuk V.I. et al. Carbon: unknown and known. - Donetsk: UK Center, 2006. (Russian)
- Bukharkina T.V. Chemistry of natural energy carriers and carbon materials / T.V. Bukharkina, N.G. Digurov. — Moscow: Russian Chemical Technical University named after. D. I. Mendeleeva, 1999. - 195 p. — ISBN 5-7237-0139-8.
- Ola D. A. Chemistry of hypercoordinated carbon. - Moscow: Mir, 1990. - 336 p. — ISBN 5-03-001451-9
| Periodic table of chemical elements by D. I. Mendeleev | |||||||||||||||||||||||||||||||||||||||||||
| C | |||||||||||||||||||||||||||||||||||||||||||
| Uue | Ubn | Ubu | Ubb | Ubt | Ubq | UBP | Ubh | ||||||||||||||||||||||||||||||||||||
| Alkali metals | Alkaline earth metals | Lanthanides | Actinoids | Superactinoids | Transition metals | Other metals | Semimetals | Other non-metals | Halogens | Noble gases | Properties unknown | ||||||||||||||||||||||||||||||||
Carbonyls.
Carbon monoxide is capable of reacting directly with many metals or metal ions, forming complex compounds called carbonyls, for example Ni(CO)4, Fe(CO)5, Fe2(CO)9, [Fe(CO)4]3, Mo(CO )6, [Co(CO)4]2. The bonding in these compounds is similar to the bonding in the cyano complexes described above. Ni(CO)4 is a volatile substance used to separate nickel from other metals. The deterioration of the structure of cast iron and steel in structures is often associated with the formation of carbonyls. Hydrogen can be part of carbonyls, forming carbonyl hydrides, such as H2Fe(CO)4 and HCo(CO)4, which exhibit acidic properties and react with alkali:
H2Fe(CO)4 + NaOH → NaHFe(CO)4 + H2O
Carbonyl halides are also known, for example Fe(CO)X2, Fe(CO)2X2, Co(CO)I2, Pt(CO)Cl2, where X is any halogen (see also ORGANOMETALLIC COMPOUNDS).