Why do metal properties weaken from left to right?
So. There are three particles in an atom: protons, neutrons and electrons. Protons have a charge of +1, electrons have a charge of -1. Neutrons have no charge.
Protons and neutrons are found in the nucleus. Therefore, the nuclear charge is always positive. And electrons spin around the nucleus and are attracted to it because they have a negative charge.
Electrons rotate through electronic levels - like planets in orbit around the Sun.
The atomic number shows how many protons there are in an atom. As you can see, this number is constantly increasing.
The more protons, the more strongly they “pull” electrons toward themselves. Compare:
Conclusion - the more protons there are, the stronger they hold electrons. The more difficult it becomes to give away these electrons. Therefore, from left to right, with increasing atomic number (and, accordingly, the number of protons), metallic properties weaken, and non-metallic properties increase.
Note - about the radius of the atom
The more protons attract electrons, the closer those electrons become to protons. Therefore, the radius of the atom decreases, the atom seems to shrink due to an increase in charge.
How to find metals and non-metals
Determination of metals by theoretical method
Theoretical method:
- All metals, with the exception of mercury, are in a solid state of aggregation. They are flexible and bend without problems. Also, these elements have good thermal and electrical conductive properties.
- If you need to determine a list of metals, then draw a diagonal line from boron to astatine, below which the metal components will be located. These also include all elements of side chemical groups.
- In the first group, the first subgroup contains alkaline ones, for example, lithium or cesium. When dissolved, we form alkalis, namely hydroxides. They have an electronic configuration of the ns1 type with one valence electron, which, when given away, leads to the manifestation of reducing properties.
The second group of the main subgroup contains alkaline earth metals such as radium or calcium. At ordinary temperatures they have a solid state of aggregation. Their electronic configuration is ns2. Transition metals are located in secondary subgroups. They have variable oxidation states. In lower degrees basic properties are manifested, intermediate degrees reveal acidic properties, and in higher degrees amphoteric properties.
Theoretical definition of non-metals
First of all, such elements are usually found in a liquid or gaseous state, sometimes in a solid state . When you try to bend them, they break due to their fragility. Nonmetals are poor conductors of heat and electricity. Nonmetals are found at the top of the diagonal line drawn from boron to astatine. Nonmetal atoms contain a large number of electrons, which makes it more profitable for them to accept additional electrons than to give them away. Non-metals also include hydrogen and helium. All non-metals are located in groups from the second to the sixth.
Chemical methods of determination
There are several ways:
- It is often necessary to use chemical methods for determining metals. For example, you need to determine the amount of copper in an alloy. To do this, apply a drop of nitric acid to the surface and after a while steam will appear. Blot the filter paper and hold it over the ammonia flask. If the spot turns dark blue, this indicates the presence of copper in the alloy.
- Let's say you need to find gold, but you don't want to confuse it with brass. Apply a concentrated solution of nitric acid to the surface in a ratio of 1 to 1. Confirmation of a large amount of gold in the alloy will be the absence of reaction to the solution.
- Iron is considered a very popular metal. To determine it, you need to heat a piece of metal in hydrochloric acid. If it is really iron, then the flask will turn yellow. If chemistry is a rather problematic topic for you, then take a magnet. If it is really iron, then it will be attracted to the magnet. Nickel is determined using almost the same method as copper, only add dimethylglyoxin to the alcohol. Nickel will confirm itself with a red signal.
Other metal elements are determined using similar methods. Just use the necessary solutions and everything will work out.
Why do nonmetallic properties weaken from top to bottom?
Go ahead. The period in the table shows the number of levels (those orbits) in which electrons fly.
The longer the period, the more of these orbits and the further the electrons are from the nucleus. Compare:
Who has a harder time keeping electrons in the last level? Copper, of course, because these electrons are twice as far from the nucleus as, for example, lithium. It becomes easier to give them away than to try to hold on to them.
Consequently, “from top to bottom” the number of levels through which electrons move increases, it becomes more difficult to retain them, therefore metallic properties become stronger, and non-metallic ones weaken.
More about the radius
If you look at the table from top to bottom, the radius of the core increases because there are more levels.
Yes, the charge of the atom also increases, but still the distance outweighs it. The more levels, the harder it becomes to hold electrons, even though the charge increases.
To summarize:
- From left to right in the table, metallic properties weaken, non-metallic properties increase due to the fact that the nucleus attracts electrons more strongly to itself.
- From top to bottom, metallic properties increase, non-metallic properties weaken, because there are more levels, and it becomes more difficult to retain electrons at the last levels.
From these two provisions it follows that non-metals will be concentrated in the right corner of the periodic table, and metals in the left.
I found you this picture, it shows all the non-metals. What is in italics are the so-called metalloids - they seem to be not quite metals, and at the same time not non-metals. Something in between.
This is the basics. I hope I explained it clearly and you understood it. If not, re-
Metal alloys
In addition, in industry there are also metal alloys that were obtained by fusing a metal with non-metals or other metals, for example, cast iron, steel, bronze, brass.
Alloys can be made from two or more components. However, not all components interact well with each other, so it is not always possible to obtain the desired alloy. For example, iron and lead, lead and zinc do not fuse with each other, since in the liquid state they do not form a solution.
A prerequisite for obtaining alloys is the formation of a homogeneous liquid solution. The resulting alloys have properties different from those of the components from which they were formed.
Pure metals are used extremely rarely in industry, since they do not always have the required properties and efficiency.
There is another way to distinguish metals from non-metals: a magnet. However, it should be noted that a magnet is a limited means of identifying metals, since only base metals have the properties of attracting to it. So, for example, cast iron, steel, and iron will be attracted to a magnet, but aluminum, silver, and copper will not be attracted. In the same way, you cannot test gold at home for authenticity.
Video on how to distinguish metals
How to distinguish slag from metal?
Slags are by-products that are obtained as a result of the following processes:
- Melting of non-ferrous and ferrous metals.
- Combustion of solid fuel.
- Electrothermal sublimation of phosphorus.
Metallurgical slags are melts that cover liquid metal during the metallurgical process. After hardening, the slags are stone-like or glassy substances.
The mineral and chemical composition of slag depends on the following factors:
- Composition of gangue ore.
- Fuel.
- Type of metal being smelted.
- Features of metallurgical processes.
- Fuel combustion conditions.
- Slag cooling conditions.
Slag is characterized by its physical properties:
- Melting point.
- Temperature range of solidification.
- Heat capacity.
- Viscosity.
- Ability to dissolve sulfides, oxides, etc.
- A certain density.
- Certain gas permeability.
The optimal melting temperature of slag is 1100-1200 °C. If steel melts at a temperature of 1400-1500 °C, then the slag must have low viscosity, high mobility and fluidity - these conditions ensure the correct formation of the weld in welding operations. The way the molten slag solidifies plays a very important role. Slags do not have a strictly defined temperature melting regime. If the temperature rises, the slag becomes less viscous, and if it decreases, the viscosity increases.
The composition and properties of slag depend on the original fluxes. The temperature of the metal under flux must be at least 1500-1550 °C, and the temperature of the slag - 1750 °C.
The question often arises of how to distinguish slag from metal. The main differences are:
- Metal has greater fluidity and mobility.
- When melted, you can see how the metal boils, which cannot be said about slag.
- Slags are more viscous and have a darker color compared to metal.
- Slags always weigh less than metals.
What are semimetals
In the periodic table, between metals and non-metals, there are a number of chemical elements that occupy an intermediate position. They are called semimetals. Semimetal atoms are linked by covalent chemical bonds.
These substances combine the characteristics of metals and non-metals. For example, antimony is a silvery-white crystalline substance that reacts with acids to form salts—typical metallic properties. On the other hand, antimony is a very fragile substance that cannot be forged, and it can even be crushed by hand.
So, typical non-metals and metals have opposite properties, but this division is quite arbitrary, since a number of substances combine both characteristics.
Physical properties
Let's start with the state of aggregation. It is traditionally believed that all metals are solids. The only exception is mercury, a viscous silvery liquid. Its vapors are a contaminant - a toxic substance that causes poisoning of the body.
Another characteristic feature is a metallic luster, which is explained by the fact that the metal surface reflects light rays. Another important feature is electrical and thermal conductivity. This property is due to the presence of free electrons in metal lattices, which begin to move directionally in an electric field. Mercury conducts heat and current best of all, silver has the lowest performance.
The metal bond determines malleability and ductility. According to these indicators, gold is the leader, from which you can roll out a sheet as thick as a human hair.
Most often, the physical properties of metals and non-metals are opposite. Thus, the latter are characterized by low electrical and thermal conductivity and lack of metallic luster. Under normal conditions, nonmetals are in a gaseous or liquid state, while solids are always brittle and fusible, which is explained by the molecular structure of nonmetals. Diamond, red phosphorus and silicon are refractory and non-volatile; they are substances with a non-molecular structure.
Simple substances - non-metals - GDZ Gabrielyan Sladkov 8th grade workbook
PART 1
1. Non-metals (NM) are located in groups III-VII. The group of halogens consists only of NM. According to their physical properties, NMs should also include group VIIIA, or the group of noble gases.
2. Non-metal atoms have 4 or more electrons in the outer layer, a small radius of an atom, for example C, whose atom has 4 outer e. Therefore, NM atoms strive to bring the missing ones to 8e. This property of atoms is characterized by electronegativity. In accordance with it, NMs form a special series:
3. Molecules of simple substances NM are formed due to covalent nonpolar bonds. Diatomic molecules have, for example, the following simple substances: O₂, Cl₂, N₂, halogens; triatomic molecule O₃ - in ozone.
4. Allotropy is more typical for nonmetals than for metals. Fill out the table “Allotropic modifications of non-metals”. Find data for the table using additional sources of information, including the Internet.
5. Fill out the table “Comparison of the properties of metals and non-metals.”
PART 2
1. Select the names of simple substances - non-metals. From the letters corresponding to the correct answers, you will form the name of the non-metal, which translated from Greek means “death, destruction”: fluorine.
1) bromine F 2) magnesium T 6) neon O iodine R
iodine R
2. Distribute the substances Na, Br2, Ne, I2, Li, He, Cl2 into three groups. Calculate and record the relative molecular weights of the halogens.
3. The following statements characterizing non-metals are incorrect:
2) under normal conditions, fluorine, chlorine and bromine are gases. 4) atoms of these elements tend to give up electrons from the outer energy layer.
4. Establish a correspondence between the name of the substance and its properties.
5. Fill out the table “Non-metals”. Find data for the table using additional sources of information, including the Internet.
6. Arrange the following simple substances - nonmetals in order of increasing density.
6) silicon 7) red phosphorus 1) iodine 2) bromine 4) chlorine 3) nitrogen 5) hydrogen
7. Arrange the following simple substances - non-metals in order of increasing intensity of their color.
6) iodine 4) bromine 1) chlorine 3) ozone 2) oxygen 5) nitrogen
Source
How do metals differ from each other?
Many people do not know how metals differ from metals. Their differences can be classified:
- Metals differ in color from each other, such as gold and copper.
- Metals also melt at different temperatures. Some metals, such as tin and lead, can be melted at home, but others require higher temperatures.
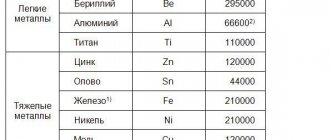
- Metals are divided into two groups: heavy and light. Heavy metals include those whose density is from 5 g/cm3, light metals have a density less than 5 g/cm3. Light metals include lithium, which has a density of 0.2 g/cm3; the place of the heaviest metal is shared by osmium and iridium. Their density is 22.6 g/cm3.
- Metals differ from each other in ductility and electrical conductivity. Some of them are very flexible. For example, from just 1 gram of gold you can make a thin wire of 3.5 kilometers. It will be flexible and will not break. It will not be possible to repeat this with a less ductile metal.
- Also, some metals conduct current better than others. The most electrically conductive metals are copper, silver and aluminum. They are most often used as conductive elements.