General information
Zirconium (Zr) is an element of the periodic table whose atomic number is 40 and its atomic weight is 91.22. In normal condition and under normal conditions, this material is a shiny metal with a silvery-white tint. The density of such raw materials reaches 6.45 g/cm3. This metal in its pure form, containing no impurities, is distinguished by the fact that it has very high ductility and is very easy to process, both cold and hot. It is worth noting here that this raw material, like titanium, for example, will sharply lose its mechanical properties if it is combined with impurities of non-metallic substances. The worst combination is zirconium and oxygen.
Material and alloy properties
Zirconium itself stands out because it has a fairly high resistance to various acids. This raw material does not dissolve in environments such as nitric and hydrochloric acid or alkalis. This characteristic is key. Based on it, many zirconium alloys are created. For example, if you take multicomponent magnesium alloys and add an element such as zirconium to them, the material will become much more resistant to corrosion. If you create an alloy of titanium and zirconium, the acid resistance of the first element will increase.
It is also worth noting that all zirconium alloys with other metals are characterized by the fact that they do not lose their viscosity over a wide temperature range; resistance to mechanical impact loads remains at a very high level. An example can be given of an alloy of magnesium with a few percent of zinc and only a few tenths of a percent of zirconium. The resulting metal will be almost twice as strong as simple magnesium, and it will also be able to maintain its strength at temperatures up to 200 degrees Celsius.
Method for producing aluminum alloys with lead
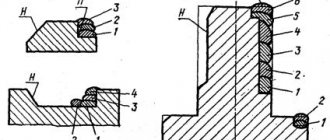
Use: for producing aluminum-lead alloys with a given size of lead phase inclusions. The goal is to increase the efficiency of using the new antifriction alloy. Essence: the production of aluminum-lead alloys by aluminothermic reduction of lead from its oxide is carried out by introducing lead oxide into a layer of graphite powder on the surface of the melt or into a refractory mesh located on the graphite powder above the melt. 2 tables
The invention relates to non-ferrous metallurgy and can be used in the production of aluminum-lead pseudo-alloys.
There is a known method for producing aluminum alloys with lead, in which the alloy is produced by introducing tin into the aluminum melt and then adding metallic lead. When producing an alloy in this way, the method is limited by the possibility of introducing only a few percent of lead. The known method does not make it possible to regulate the size of inclusions of the lead phase in the resulting alloy. But the practice of using such alloys dictates the need for such regulation, since the size of the lead phase in the resulting alloy affects the antifriction characteristics. The optimal values of the size of the lead phase can vary widely depending on the load, sliding speeds and other friction modes. The purpose of the invention is to obtain aluminum-lead alloys with a Pb content of up to 30% or more with specified limits for the size of inclusions of the lead phase. The goal is for practically necessary lead content and the range of sizes of the lead phase is achieved by the fact that the production of an aluminum-lead alloy in an aluminum melt with 1.0% tin is carried out by introducing lead oxide into a layer of graphite powder on the surface of the melt or into a refractory mesh located on the graphite powder above the melt. the alloy formation process using graphite powder of a specified granulometric composition instead of liquid flux ensures the production of alloys with varying dispersion of the lead phase in the range of up to 30-40 microns. When installing a refractory mesh on a layer of graphite powder, the size of the holes in this mesh allows you to change the size of inclusions of the lead phase in the resulting alloy to 120 μm. EXAMPLE. Graphite powder is distributed in a uniform layer on the surface of the aluminum melt with 1.0% tin. After heating it, lead oxide powder is introduced. The introduction is carried out gradually as the additive is absorbed in the required amount (up to 30% or more). After this, the resulting melt was poured into a mold and the ingot was subjected to chemical and metallographic analysis. The results of the experiments are presented in Table 1. In the second series of experiments, a ceramic or graphite mesh was installed on a small layer of graphite powder distributed on the surface of an aluminum melt with 1.0% tin. Lead oxide was introduced onto this grid in one portion. After heating the powder, the mesh was rotated at low speed. Upon completion of the reaction of assimilation of lead oxide, which could be detected by the cessation of point glow in the additive, the resulting melt was poured into a mold and the ingot was subjected to chemical and metallographic analysis. The experimental results are presented in Table 2. The proposed method makes it possible to obtain aluminum-lead alloys with a Pb content of up to 30% or more with a given size of lead phase inclusions and thereby increase the efficiency of using the new antifriction alloy.
Claim
1. METHOD FOR PRODUCING ALLOYS OF ALUMINUM WITH LEAD, including the introduction of tin and lead additives into the aluminum melt, characterized in that the introduction of tin is carried out in an amount of 10% by weight of aluminum, its oxide is used as a lead additive, and the introduction of lead oxide is carried out in a layer of powder graphite on the surface of the melt or in a refractory mesh located on a layer of graphite powder.
DRAWINGS
,
Description of characteristics
Zirconium alloys are most actively used in such areas as fuel rod cladding, fuel channel pipes, and various fuel assembly parts. Zirconium itself is also characterized by the fact that the neutron absorption cross section is quite low. In this indicator, it is second only to substances such as magnesium and beryllium. In addition, the melting point of zirconium is very high.
Zirconium alloys, used in various industries, are characterized by the fact that they have very high corrosion resistance in water, in a steam-water mixture, in saturated and superheated steam up to a temperature of approximately 350-360 degrees Celsius. It's also worth noting that this temperature limit is expected to be raised to higher values in the near future.
Alloy parameters
The properties of zirconium alloys in terms of mechanical stability are quite high, which cannot be said about pure zirconium. It is alloying that achieves high strength of the material. For example, an alloy such as niobium (Nb) and 1% zirconium (Zr) will be characterized by the fact that the yield strength of the material at temperatures of 20, 200, 300 and 400 degrees Celsius will be equal to 200, 160, 120 and 90 MPa. This alloy is actively used for fuel cladding. And, for example, if you change the composition of a zirconium alloy with niobium, that is, increase the zirconium content to 2.5%, then the yield strength will increase to 280, 220, 200 and 180 MPa, at the same temperatures.
However, such materials also have their drawbacks. The disadvantages include the fact that the alloy with zirconium turns out to be too creeping when the temperature reaches 320-350 degrees Celsius and above. Another disadvantage is that Zr actively dissolves hydrogen, which often occurs during the corrosion process. Because of this, substances such as zirconium hydrides will be formed, which greatly reduce the ductility of the raw material, which makes the metal more brittle.
Aluminum alloys of the aluminum-magnesium system
| Brand designation | Mass fraction of elements, % | Density, kg/dm3 | ||||||||||||
| according to ND* | according to ISO 209-1 | Silicon | Iron | Copper | Manganese | Magnesium | Chromium | Zinc | Titanium | Other elements | Other items | Aluminum | ||
| Si | Fe | Cu | Mn | Mg | Cr | Zn | Ti | Every | Sum | |||||
| AMg0.5 1505 | — | 0,1 | 0,1 | 0,1 | 0,2 | 0,4- 0,8 | — | — | — | — | 0,05 | 0,1 | rest | 2,70 |
| AMg1 1510 | AlMg1 5005 | 0,30 | 0,7 | 0,20 | 0,20 | 0,50- 1,1 | 0,10 | 0,25 | — | — | 0,05 | 0,15 | Same | 2,69 |
| AMg1.5 | AlMg1.5 5050 | 0,40 | 0,7 | 0,20 | 0,10 | 1,1- 1,8 | 0,10 | 0,25 | — | — | 0,05 | 0,15 | Same | 2,69 |
| AMg2 1520 | AlMg2 5251 | 0,40 | 0,50 | 0,15 | 0,1- 0,6 | 1,8- 2,6 | 0,05 | 0,15 | 0,15 | — | 0,05 | 0,15 | Same | 2,69 |
| AMg2.5 | AlMg2.5 5052 | 0,25 | 0,40 | 0,10 | 0,10 | 2,2- 2,8 | 0,15- 0,35 | 0,10 | — | — | 0,05 | 0,15 | Same | 2,68 |
| AMg3 1530 | — | 0,5- 0,8 | 0,5 | 0,1 | 0,3-0,6 | 3,2- 3,8 | 0,05 | 0,2 | 0,1 | — | 0,05 | 0,1 | Same | 2,66 |
| — | AlMg3 5754 | 0,40 | 0,40 | 0,10 | 0,50 | 2,6- 3,6 | 0,30 | 0,20 | 0,15 | Mn+Cr: 0.10-0.6 | 0,05 | 0,15 | Same | 2,66 |
| AMg3.5 | AlMg3.5 5154 | 0,25 | 0,40 | 0,10 | 0,10 | 3,1- 3,9 | 0,15- 0,35 | 0,20 | 0,20 | Be: 0.0008, Mn + Cr: 0.10-0.50 | 0,05 | 0,15 | Same | 2,66 |
| AMg4.0 1540 | AlMg4 5086 | 0,40 | 0,50 | 0,10 | 0,20- 0,7 | 3,5- 4,5 | 0,05- 0,25 | 0,25 | 0,15 | — | 0,05 | 0,15 | Same | 2,66 |
| AMg4.5 | AlMg4.5 5083 | 0,40 | 0,40 | 0,10 | 0,40- 1,0 | 4,0- 4,9 | 0,05- 0,25 | 0,25 | 0,15 | — | 0,05 | 0,15 | Same | 2,66 |
| — | AlMg5Cr 5056 | 0,30 | 0,40 | 0,10 | 0,05- 0,20 | 4,5- 5,6 | 0,05- 0,20 | 0,10 | — | — | 0,05 | 0,15 | Same | 2,65 |
| AMg5 1550 | — | 0,5 | 0,5 | 0,1 | 0,3- 0,8 | 4,8- 5,8 | — | 0,2 | 0,02- 0,10 | Be: 0.0002-0.005 | 0,05 | 0,1 | Same | 2,65 |
| AMg6 1560 | — | 0,4 | 0,4 | 0,1 | 0,5- 0,8 | 5,8- 6,8 | — | 0,2 | 0,02- 0,10 | Be: 0.0002-0.005 | 0,05 | 0,1 | Same | 2,64 |
* GOST 1131, GOST 8617, GOST 15176, GOST 17232, GOST 18475, GOST 18482, GOST 21488, GOST 22233, GOST 23786
- In the AMg2 brand alloy, intended for the manufacture of tape used as packaging containers in the food industry, the mass fraction of magnesium should be from 1.8 to 3.2%.
Zirconium in medicine
Zirconium alloys are used quite actively in medicine. Scientists have found through experiments that even wearing simple zirconium bracelets can help in the treatment of certain diseases, and this can also improve a person’s overall level of well-being.
Today, implants (fixators) are quite often used in such areas of medicine as traumatology and maxillofacial surgery. Fixators are used for fractures, fixing the bones so that they do not move. It is in these cases that one can highlight such advantages of using zirconium alloys as: high biological compatibility (meaning the absence of allergic reactions of the human body to such an alloy or rejection), high strength characteristics of the alloy, which is very important for fixatives. It is also worth noting that the absence of rejection or allergy to such a substance meant that there was no need to repeat surgery to remove the fixative if the body suddenly began to reject the implant.
Zirconium in nuclear energy
Until the 50s of the last century, it was believed that zirconium was not suitable for use in this area. However, it was in the 50s. For the first time, a material was obtained that was completely purified from such an impurity as hafnium. After purification, pure zirconium was found to have a very small thermal neutron absorption cross section. It was this quality that became the main one and made it possible to use zirconium alloys in nuclear energy.
It is worth adding that it was not possible to use simply purified zirconium due to the fact that the corrosion resistance was too low in hot water. After this, it was decided to use zirconium-based alloys. They have proven themselves to be excellent when used in reactors with steam-water coolant, as well as in other similar aggressive environments.
Alloys containing aluminum
One of the well-known alloys where you can find aluminum is an aluminum compound with copper.
The resulting metal has a simple formula and strong bonds, thanks to which the alloy can be used in military and rocket technology, as well as in spacecraft. The use of copper in the composition helps to improve corrosion resistance. If manganese is found together with aluminum, then its presence can strengthen the alloy several times with a significant improvement in solidification parameters. It is this composition that will remain solid even at very high temperatures. Manganese and aluminum are used to create kitchen utensils, heating radiators, plumbing systems and air conditioners.
When silicon is found in the composition of an aluminum alloy, the resistance to melting of the composition is greatly reduced. Often this composition is used for the manufacture of castings, fillers for welding or soldering of aluminum.
General alloy applications
Zirconium is widely used as an alloying element. This is due to the fact that the metals to which this substance is added become more heat-resistant, acid-resistant, etc. That is, the alloy of metal and zirconium greatly exceeds the characteristics of the initial raw materials.
Ferrozirconium is quite widely used. It is an alloy of zirconium and iron. The content of the alloying element Zr reaches 20% of the total mass. This substance is used in metallurgy as a deoxidizer and degasser for steel. Aluminum-zirconium alloys, for example, are considered the most resistant to corrosion and are used in cathode grids for vacuum tubes. The Zr content in such an alloy is no more than 3% of the total mass.
In ferrous metallurgy, in addition to ferrozirconium, an alloy of Zr and silicon is often used. It is used for degassing steel. An alloy of copper and zirconium is widely used for the manufacture of conductive elements for electrical equipment.
Grades and chemical composition of aluminum
| Brand designation | Mass fraction of elements, % | Density, kg/dm3 | ||||||||||||
| according to ND* | according to ISO 209-1 | Silicon | Iron | Copper | Manganese | Magnesium | Chromium | Zinc | Titanium | Other elements | Other items | Aluminum no less | ||
| Every | Sum | |||||||||||||
| Si | Fe | Cu | Mn | Mg | Cr | Zn | Ti | |||||||
| AD000 | Al99,8 1080A | 0,15 | 0,15 | 0,03 | 0,02 | 0,02 | — | 0,06 | 0,02 | — | 0,02 | — | 99,80 | 2,70 |
| AD00 1010 | Al99,7 1070A | 0,20 | 0,25 | 0,03 | 0,03 | 0,03 | — | 0,07 | 0,03 | — | 0,03 | — | 99,70 | 2,70 |
| AD00E 1010E | EAl99,7 1370 | 0,10 | 0,25 | 0,02 | 0,01 | 0,02 | 0,01 | 0,04 | — | B: 0.02, V+Ti: 0.02 | 0,02 | 0,10 | 99,70 | 2,70 |
| — | Al99,6 1060 | 0,25 | 0,35 | 0,05 | 0,03 | 0,03 | — | 0,05 | 0,03 | V:0.05 | 0,03 | — | 99,60 | 2,70 |
| AD0 1011 | Al99,5 1050A | 0,25 | 0,40 | 0,05 | 0,05 | 0,05 | — | 0,07 | 0,05 | — | 0,03 | — | 99,50 | 2,71 |
| AD0E 1011E | EAl 99,5 1350 | 0,10 | 0,40 | 0,05 | 0,01 | — | 0,01 | 0,05 | — | B: 0.05 V+Ti: 0.02 | 0,03 | 0,10 | 99,50 | 2,71 |
| AD1 1013 | A l 99.3 | 0,3 | 0,3 | 0,05 | 0,025 | 0,05 | — | 0,1 | 0,15 | — | 0,05 | — | 99,30 | 2,71 |
| HELL 1015 | Al99,0 1200 | Si+Fe: 1.0 | — | 0,1 | 0,1 | — | — | 0,10 | 0,15 | — | 0,05 | 0,15 | 99,0 | 2,71 |
| AD1pl | — | 0,30 | 0,30 | 0,02 | 0,025 | 0,05 | — | 0,1 | 0,15 | — | 0,02 | — | 99,30 | 2,71 |
* GOST 1131, GOST 8617, GOST 15176, GOST 17232, GOST 18475, GOST 18482, GOST 21488, GOST 22233, GOST 23786.
- “E” is used to designate a grade of aluminum with guaranteed electrical characteristics
- The actual aluminum content of unalloyed aluminum is determined by the difference between 100% and the sum of all elements present in amounts of 0.010% or more each, expressed to the second decimal place.
- When determining the grade of aluminum, the content of titanium introduced as a modifier should not be taken into account in the total amount of impurities.
- It is allowed to set the copper content in the AD1pl alloy to 0.05%.
- In aluminum grade AD0 for sheet blanks subjected to further molding, the introduction of titanium up to 0.15% is allowed.
- The ratio of iron and silicon in aluminum must be at least unity.
Aluminum alloys of the aluminum-copper-magnesium and aluminum-copper-manganese systems
| Brand designation | Mass fraction of elements, % | Density, kg/dm3 | |||||||||||||
| according to ND* | according to ISO 209-1 | Silicon | Iron | Copper | Manganese | Magnesium | Chromium | Zinc | Titanium | Nickel | Other elements | Other items | Aluminum | ||
| Every | Sum | ||||||||||||||
| Si | Fe | Cu | Mn | Mg | Cr | Zn | Ti | Ni | |||||||
| D1 1110 | AlCu4MgSi 2017 | 0,20- 0,8 | 0,7 | 3,5- 4,8 | 0,40- 1,0 | 0,40- 0,8 | 0,10 | 0,3 | 0,15 | — | Ti+Zr: 0.20 | 0,05 | 0,15 | rest | 2,80 |
| D16 1160 | AlCuMgl 2024 | 0,50 | 0,50 | 3,8- 4,9 | 0,30- 0,9 | 1,2- 1,8 | 0,10 | 0,25 | 0,15 | — | Ti+Zr: 0.20 | 0,05 | 0,15 | Same | 2,77 |
| D16h | 2124 | 0,20 | 0,30 | 3,8- 4,9 | 0,30- 0,9 | 1,2- 1,8 | 0,10 | 0,25 | 0,15 | — | — | 0,05 | 0,15 | Same | 2,78 |
| B65 1165 | — | 0,25 | 0,2 | 3,9- 4,5 | 0,3- 0,5 | 0,15- 0,30 | — | 0,1 | 0,1 | — | — | 0,05 | 0,1 | Same | 2,80 |
| D18 1180 | AlCu2.5Mg 2117 | 0,5 | 0,5 | 2,2- 3,0 | 0,20 | 0,20- 0,50 | 0,10 | 0,1 | — | — | — | 0,05 | 0,15 | Same | 2,74 |
| D19 1190 | — | 0,5 | 0,5 | 3,8- 4,3 | 0,5- 1,0 | 1,7- 2,3 | — | 0,1 | 0,1 | — | Be: 0.0002-0.005 | 0,05 | 0,1 | Same | 2,76 |
| AK4 1140 | — | 0,5- 1,2 | 0,8- 1,3 | 1,9- 2,5 | 0,2 | 1,4- 1,8 | — | 0,3 | 0,1 | 0,8- 1,3 | — | 0,05 | 0,1 | Same | 2,77 |
| AK4-1 1141 | — | 0,35 | 0,8- 1,4 | 1,9- 2,7 | 0,2 | 1,2- 1,8 | 0,1 | 0,3 | 0,02- 0,10 | 0,8- 1,4 | — | 0,05 | 0,1 | Same | 2,80 |
| AK4-1ch | 2618 | 0,10- 0,25 | 0,9- 1,3 | 1,9- 2,7 | — | 1,3- 1,8 | — | 0,10 | 0,04- 0,10 | 0,9- 1,2 | — | 0,05 | 0,15 | Same | 2,80 |
| 1201 | AlCu6Mn 2219 | 0,20 | 0,30 | 5,8- 6,8 | 0,20- 0,40 | 0,02 | — | 0,10 | 0,01- 0,10 | — | Zr: 0.10-0.25, V: 0.05-0.15 | 0,05 | 0,15 | Same | 2,85 |
| AK6 1360 | — | 0,7- 1,2 | 0,7 | 1,8- 2,6 | 0,4- 0,8 | 0,4- 0,8 | — | 0,3 | 0,1 | 0,1 | — | 0,05 | 0,1 | Same | 2,75 |
| AK8 1380 | AlCu4SiMg 2014 | 0,50- 1,2 | 0,7 | 3,9- 5,0 | 0,40- 1,0 | 0,20- 0,8 | 0,10 | 0,25 | 0,15 | — | Ti+Zr: 0.20 | 0,05 | 0,15 | Same | 2,80 |
| 1105 | — | 3,0 | 1,5 | 2,0- 5,0 | 0,3- 1,0 | 0,4- 2,0 | — | 1,0 | — | 0,2 | Ti+Cr+Zr: 0.2 | 0,05 | 0,2 | Same | 2,80 |
*GOST 1131, GOST 8617, GOST 15176, GOST 17232, GOST 18475, GOST 18482, GOST 21488, GOST 22233, GOST 23786
- The amount of titanium + zirconium is limited only for extruded and forged semi-finished products and only if there is an agreement between the manufacturer and the consumer.
Aluminum alloys of the aluminum-manganese system
| Brand designation | Mass fraction of elements, % | Density, kg/dm3 | |||||||||||
| according to ND* | according to ISO 209-1 | Silicon | Iron | Copper | Manganese | Magnesium | Chromium | Zinc | Titanium | Other items | Aluminum | ||
| Every | Sum | ||||||||||||
| Si | Fe | Cu | Mn | Mg | Cr | Zn | Ti | ||||||
| MM 1403 | AlMnMg0.5 3005 | 0,6 | 0,7 | 0,30 | 1,0- 1,5 | 0,20- 0,6 | 0,10 | 0,25 | 0,10 | 0,05 | 0,15 | rest | 2,72 |
| AMts 1400 | AlMn1Cu Al 3003 | 0,6 | 0,7 | 0,2 | 1,0- 1,5 | 0,2 | — | 0,10 | 0,1 | 0,05 | 0,15 | Same | 2,73 |
| AMtsS 1401 | — | 0,15- 0,35 | 0,25- 0,45 | 0,1 | 1,0- 1,4 | 0,05 | — | 0,1 | 0,1 | 0,05 | 0,1 | Same | 2,73 |
| D12 1521 | AlMn1Mg1 3004 | 0,30 | 0,7 | 0,25 | 1,0- 1,5 | 0,8- 1,3 | — | 0,25 | — | 0,05 | 0,15 | Same | 2,72 |
* GOST 1131, GOST 8617, GOST 15176, GOST 17232, GOST 18475, GOST 18482, GOST 21488, GOST 22233, GOST 23786.
- It is allowed to introduce up to 0.2% titanium into AMts grade aluminum for sheet blanks subjected to further molding.
- The ratio of iron and silicon in the AMtsS alloy should be greater than unity.