Physical parameters of aluminum and melting point
The melting point of aluminum characterizes the gradient of transition to the liquid state and determines the physical parameters of the chemical element.
The properties of the metal make it possible to use it in various branches of industrial production, and the ability to form stable compounds significantly expands the scope of its use. The ability to change from solid to liquid determines the physical properties of a metal.
Characteristics of physical and technical parameters of aluminum
- Aluminum is one of the most common chemical elements and is characterized by light weight and softness. The basic physical parameters of the metal, the ability to form compounds resistant to environmental influences, allow it to be used in various branches of industrial production.
- Metal is an attractive material for working at home. The specific heat of fusion of aluminum is 390 kJ/kg, and for foundry purposes it is not difficult to melt it at home.
- Melting of metal can be carried out by surface and internal heating. The method of external thermal influence does not require special equipment and is used in artisanal conditions.
- Aluminum, the melting point of which depends on the purity of the compound and pressure, requires heating to an average of 660 °C or 993.5 °K to transform into a liquid state.
- There are different opinions regarding the melting temperature of metal at home, but they can only be verified in practice.
Properties of metal alloys
The temperature gradient indicator fluctuates for metal compounds with other chemical elements that determine their properties. For cast alloys containing magnesium and silicon, it is 500 °C.
The specific heat of fusion determines the physical property of a chemical element. For alloys, this indicator characterizes the process of transition from one state of aggregation to another in a certain temperature range.
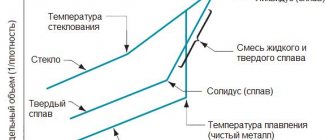
The temperature at which the transition to the liquid state begins is called the solidus (solid) point, and the end point is called the liquidus (liquid) point. Accordingly, the beginning of crystallization will be determined by the liquidus point, and the end - by the solidus. In the temperature range, the compound is in a transition state from liquid to solid phase.
In some compounds of aluminum with other chemical elements, there is no interval between the temperature indicators of the transition from solid to melt. These alloys are called eutectic .
For example, a compound of aluminum with 12.5% silicon, like pure metal, has a melting point, not a range. This alloy is a casting alloy and is characterized by a constant temperature of 577 °C.
As the amount of silicon in the alloy increases, the liquidus gradient decreases from the maximum characteristic of pure metal. Among alloying additives, the temperature gradient reduces the use of magnesium (450 °C). For a connection with copper it is 548 °C, and with manganese it is only 658 °C.
Aluminum forms various alloys with minerals.
Most compounds consist of several components, which affects the rate of solidification and melting of the material. The concepts of temperature gradients solidus and liquidus are defined for the infinite duration of processes of equilibrium transitions into the liquid and solid states.
In practice, corrections to the heating and cooling rates of the compositions are taken into account.
Application of metal in industrial production
Under natural conditions, aluminum tends to form a thin oxide film, which prevents reactions with water and nitric acid (without heating). When the film is destroyed as a result of contact with alkalis, the chemical element acts as a reducing agent.
In order to prevent the formation of an oxide film, other metals (gallium, tin, indium) are added to the alloy. The metal is practically not subject to corrosion processes. It is a popular material in various industries.
Aluminum and its alloys are in great demand in various spheres of human life.
- Aluminum is considered a popular material for making tableware and the main raw material for the aviation and space industries. The excellent electrical conductivity of the metal allows it to be used for sputtering conductors in microelectronics.
- The property of aluminum and its alloys to become brittle at low temperatures allows it to be used in cryogenic technology. Reflectivity and low cost, ease of vacuum deposition make aluminum an indispensable material for the manufacture of mirrors.
- The application of metal to the surface of parts of turbines and oil platforms makes steel alloys resistant to corrosion. Metal sulfide is used to produce hydrogen sulfide, and pure aluminum is used as a reducing agent for rare alloys from oxides.
- The chemical element is used as a component of compounds, for example, in aluminum bronzes and magnesium alloys. Along with other materials, it is used for the manufacture of spirals in electric heating devices. Metal compounds are widely used in glass making.
- Currently, pure aluminum is rarely used as a material for jewelry, but its alloy with gold, which has a special shine and sparkle, is gaining popularity. In Japan, metal is used instead of silver to make jewelry.
- Aluminum is registered as an additive in the food industry. Aluminum beer cans have been a popular beverage packaging since the 1960s. The technological line provides for the production of containers of 0.33 and 0.5 liters. The packaging has the same diameter and differs only in height.
- The main advantage of packaging over glass is the possibility of recycling the material.
- Cans for beer (carbonated drinks) can withstand pressure up to 6 atmospheres, have a dome-shaped, thick bottom and thin walls. Features of the manufacturing technology by drawing ensure the structural strength and reliable performance properties of the container.
Combustion of metals and alloys
⇐ PreviousPage 21 of 68Next ⇒
The ability to burn alkali and alkaline earth metals (potassium, sodium, lithium, magnesium, etc.) is well known. However, it is less known that in certain situations, incl. Under certain fire conditions, metals and alloys that are not usually considered flammable can burn. The most common of these include various aluminum-based alloys, widely used in construction, mechanical engineering and other fields.
As is known, the resistance of aluminum to oxidation is due to the presence on its surface of a thin (about 0.0002 mm), very dense and non-porous oxide film. However, aluminum heated in air to a temperature close to the melting point (660 0C) still begins to oxidize further, and the oxidation rate increases significantly as the temperature rises above the melting point. It should be noted that the reaction of aluminum with oxygen is exothermic and is accompanied by a significantly greater release of heat than the oxidation reaction of other metals (1675 kJ/mol) [93].
The oxidation of aluminum is enhanced by the presence of impurities of magnesium, calcium, sodium, silicon, and copper. Aluminum-magnesium alloys are especially easily oxidized when heated, on the surface of which loose oxide films are formed [94].
Table 1.19 shows the auto-ignition temperatures in air of aluminum-magnesium alloys with different magnesium contents in the alloy.
Table 1.19
Self-ignition temperatures of aluminum-magnesium alloys
in air (powders 0-50 microns, DTA) [94]
| Contents Mg in alloy, wt.% | 9,1 | 15,5 | 20,0 | 28,0 | 34,8 | 45,4 | 49,9 | 61,6 | 75,0 | 85,0 | 90,0 | 95,0 |
| T self-igniting, 0C | does not burn |
It is interesting to note that the auto-ignition temperature does not decrease monotonically with increasing Mg content from 0 to 100%; Alloys containing approximately equal parts of Mg and Al have extremely low auto-ignition temperatures.
Of course, the data presented characterize the properties of alloys in finely dispersed form. As is known, the tendency of a metal (alloy) to ignite and the ignition temperature strongly depend on its state of aggregation - the more dispersed the metal, the larger the surface of its contact with air, the easier it is to heat each particle to a critical temperature and the easier the oxidation process occurs, up to spontaneous combustion. And yet, in large fires, with large heat flows, there have been cases where not only metals and alloys in a crushed state burned, but also literally metal structures. Firefighters observed such things, for example, during the burning of warehouses made of light metal structures (aluminum alloys) with combustible (polyurethane foam) insulation.
The environment can play a special role here. An increased oxygen content sharply increases the possibility of ignition and the intensity of combustion of any material, including metal (alloy). This is well known to specialists from descriptions of fires on submarines, in medical oxygen therapy chambers, and in industries associated with the consumption of gaseous and (which is especially dangerous) liquid oxygen.
It is widely known that combustion can occur when mineral oil enters an oxygen cylinder, hose, pipeline due to spontaneous combustion of the latter. It is much less known that combustion can occur as a result of friction of parts in an oxygen atmosphere: when opening and closing valves and gates, actuation of valves and switching devices, adjustment of gearboxes, at the time of starting and stopping machines [95-98]. It is not only the friction of metal on metal that is dangerous here; when shut-off valves are activated or valves are suddenly opened, a high-speed flow of oxygen occurs, accompanied by the formation of compression waves, shock waves and a sharp increase in oxygen pressure and temperature [99]. Of course, these processes, as a rule, do not provide the release of thermal energy sufficient to ignite the metal and alloy directly. In practice, the ignition of the latter occurs through the chain: “heat generation - ignition of non-metallic materials, fatty substances or deposits - ignition of metal.” Non-metallic materials and products of this kind include gaskets made of paronite, fiber, rubber, and fluoroplastic. Fire can occur when welding bead or mill scale enters the oxygen current [95, 100].
The tendency of various metals and alloys to burn in a flow of oxygen can be judged from the data in Table. 1.20.
Table 1.20
Limit oxygen pressures at which
combustion of various metals is possible [95]
(sample thickness - 3 mm, temperature - 20 0C,
the sample is positioned horizontally)
| Metal (alloy) | P, MPa |
| Steel St3, St10 | 0,02 |
| Aluminum, AMC, AMG alloys | 0,1 |
| Cuprous cast iron | 1,1 |
| Stainless steel (13% Cr, 19% Mn) | 1,5 |
| Steel 3 ´ 13 | 2,2 |
| Stainless steel steel X18N10T | 2,6 |
| Copper, brass, nickel | > 4,2 |
From the data presented it follows that the most common grades of structural steel (low-carbon, unalloyed), as well as aluminum and aluminum-based alloys, are most prone to combustion in oxygen.
The rate of combustion of metals in oxygen depends on the geometric dimensions of the product and oxygen pressure. As the size and thickness of the product increases, the speed naturally decreases; with increasing pressure it increases. An idea of the absolute values of combustion rates is given by the information given in Table 1.21.
Table 1.21
Burning rates of metals and alloys in oxygen
At gas pressure 1-10 MPa
(samples 3 mm thick, horizontally located) [95]
| Metal (alloy) | U, cm/sec |
| Mild steel | 0,4-1,4 |
| Steel X18N9 | 1,2-1,7 |
| Cuprous cast iron | 0,4-1,0 |
| Alloy AMC | 6,9-11,2 |
| AMg6 alloy | 7,4 -9,9 |
Visual signs of burning of a metal (alloy) is the destruction of the structure (object) in the combustion zone. A burnt-out part often leaves an openwork “skeleton”. Combustion is accompanied by spattering , especially intense if it occurs in a gas flow. In this case, multiple small particles of frozen metal and metal oxides are found at the fire site. A similar scatter of particles occurs during the combustion of an electric arc, in which metal combustion processes take place along with melting.
The combustion of metals and alloys in a fire can make significant adjustments to the picture of thermal damage and to the formation of focal and “pseudo-focal” signs. This should be taken into account whenever possible. The tendency of a particular metal (alloy) to exothermic interaction with oxygen in the air (combustion) can be determined by an expert analytically, for example, by examining a metal sample using the DTA method. For more information about this, see Part III.
Instrumental research methods
⇐ Previous21Next ⇒
Recommended pages:
Use the site search:
Aluminum
Aluminum came into industrial and household use relatively recently. At the intersection of the 19th and 20th centuries, the production of this metal on an industrial scale was mastered.
The thing is that the production of many goods began in which aluminum was widely used, for example, in the construction of boats, railway cars, etc.
By the way, it was then that a car with a body made of aluminum was shown to the general public.
Anodized aluminum
Composition and structure of aluminum
Aluminum is the most common metal in the earth's crust. It is classified as a light metal. It has low density and mass. In addition, it has a fairly low melting point. At the same time, it has high ductility and shows good thermal and electrical conductivity characteristics.
Aluminum crystal latticeAluminum structure
The tensile strength of pure aluminum is only 90 MPa. But, if you add some substances to the melt, for example, copper and a number of others, then the tensile strength increases sharply to 700 MPa. The same result can be achieved using heat treatment.
Aluminum, which has extremely high purity - 99.99%, is produced for use in laboratory purposes. For industrial applications, commercially pure aluminum is used.
When producing aluminum alloys, additives such as iron and silicon are used.
They do not dissolve in the aluminum melt, and the additive reduces the ductility of the base material, but at the same time increases its strength.
Appearance of a simple substance
The structure of this metal consists of simple cells consisting of four atoms. This structure is called face-centric.
Calculations show that the density of pure metal is 2.7 kg per cubic meter.
Properties and characteristics
Aluminum is a metal with a silvery-white surface. As already noted, its density is 2.7 kg/m3. The temperature is 660°C.
Its electrical conductivity is equal to 65% of copper and its alloys. Aluminum and most of its alloys are resistant to corrosion. This is due to the fact that an oxide film forms on its surface, which protects the base material from exposure to atmospheric air.
In the untreated state, its strength is 60 MPa, but after adding certain additives it increases to 700 MPa. The hardness in this state reaches 250 HB.
Aluminum can be easily processed under pressure. To remove work hardening and restore ductility after processing, aluminum parts are annealed, and the temperature should be within 350°C.
The production of aluminum melt, like many other materials, occurs after thermal energy has been supplied to the original metal. It can be supplied either directly into it or from outside.
The melting point of aluminum directly depends on the level of its purity:
- Ultra-pure aluminum melts at a temperature of 660.3°C.
- With an aluminum content of 99.5%, the melting point is 657°C.
- With a content of this metal of 99%, the melt can be obtained at 643°C.
Aluminum meltAluminum production process
An aluminum alloy can contain various substances, including alloying ones. Their presence leads to a decrease in the melting point.
For example, if there is a large amount of silicon, the temperature can drop to 500°C. In fact, the concept of melting point applies to pure metals.
Alloys do not have any constant melting point. This process occurs within a certain heating range.
In materials science there is a concept - solidus and liquidus temperatures.
The first temperature indicates the point at which the melting of aluminum begins, and the second shows at what temperature the alloy will finally melt. In the interval between them, the alloy will be in a mushy state.
Decrease temperature
Before you start melting metal, you can perform certain operations that will reduce the melting temperature. For example, sometimes aluminum powder is melted. In a powdered state, the metal begins to melt somewhat faster.
But with such processing, there is a real danger that when interacting with oxygen contained in the atmosphere, aluminum powder will begin to oxidize with a large release of heat and the formation of metal oxides; this process occurs at a temperature of 2300 degrees.
The main thing is to prevent contact between the melt and water at this moment of melting. This will cause an explosion.
The relatively low melting point of aluminum allows this operation to be carried out at home.
It should be noted right away that using a powdered mixture as a raw material in a home workshop is too dangerous. Therefore, either ingots or cut wire are used as raw materials.
If there are no special quality requirements for the future product, then anything made from this metal can be used for melting.
Melting aluminum in a homemade forge
In this case, it is not particularly important whether the raw materials are coated with paint or not. When aluminum melts, all foreign substances will simply burn out and be removed along with the slag.
To obtain a high-quality melting result, it is necessary to use materials called fluxes. They are designed to solve the problem of binding and removing foreign impurities and contaminants from the melt.
A home craftsman who decides to melt aluminum at home should be aware that this is a rather dangerous process. And therefore it is impossible to do without the use of protective equipment.
In particular, gloves, an apron, and goggles should be used. The fact is that the melt temperature is within 600 degrees.
Therefore, it makes sense to use the protective equipment that welders use.
Using protective equipment when melting aluminum
By the way, when melting aluminum and using cleaning chemicals, it is necessary to protect the respiratory system from combustion products.
Selecting a mold for casting
When choosing a mold for casting aluminum, a home craftsman must understand for what purpose he is processing aluminum. If the future casting is intended for use as solder, then there is no need to use any special forms. To do this, you can use a metal sheet on which to cool the molten metal.
But if there is a need to obtain even a simple part, then the master must decide on the type of mold for casting.
The mold can be made from plaster. To do this, gypsum in a liquid state is poured into an oil-treated mold. After it begins to harden, a casting model is installed into it. In order for molten metal to be poured into the mold, a sprue must be formed.
To do this, a cylindrical part is placed in the mold. Forms can be detachable or not. The process of making a split mold is complicated by the fact that the model will be in two halves. After hardening, they are separated, the model is removed and connected again. The form is ready to use.
Aluminum casting die
To obtain high-quality castings, it is advisable to use metal molds (moulds), but it is advisable to produce them only in factory conditions.
, please select a piece of text and press Ctrl+Enter.
| Aluminum - chemical element of group III of the periodic system of Mendeleev (atomic number 13, atomic mass 26.98154). In most compounds, aluminum is trivalent, but at high temperatures it can also exhibit the +1 oxidation state. Of the compounds of this metal, the most important is Al2O3 oxide. Aluminum is a silvery-white metal, lightweight (density 2.7 g/cm3), ductile, a good conductor of electricity and heat, melting point 660 °C. It is easily drawn into wire and rolled into thin sheets. Aluminum is chemically active (in air it becomes covered with a protective oxide film - aluminum oxide) and reliably protects the metal from further oxidation. But if aluminum powder or aluminum foil is heated strongly, the metal burns with a blinding flame, turning into aluminum oxide.
|
Aluminum in the form of a compact material does not interact with carbon tetrachloride. Mixing aluminum dust with some chlorinated hydrocarbons and alcohol causes the mixture to spontaneously ignite. A mixture of aluminum powder with copper oxide, silver oxide, lead oxide and especially lead dioxide burns explosively. A mixture of ammonium nitrate, aluminum powder with coal or nitro compounds is an explosive. Extinguishing agents: dry sand, alumina, magnesite powder, asbestos blanket. The use of water and fire extinguishers is prohibited.
Aluminum does not occur in nature in its pure form, because it is very quickly oxidized by atmospheric oxygen to form strong oxide films that protect the surface from further interaction.
Typically, not pure aluminum is used as a structural material, but various alloys based on it, which are characterized by a combination of satisfactory strength, good ductility, very good weldability and corrosion resistance. In addition, these alloys are characterized by high vibration resistance.