Galvanic galvanizing of metal structures is one of the most popular types of galvanizing, thanks to which products acquire high protective and decorative properties.
Unlike the hot processing method, this technology is simpler and cheaper.
However, it has certain restrictions on use, which is due to the relatively thin layer of protective coating (does not exceed 40 microns).
Electroplating technology involves the deposition of zinc from the electrolyte onto a metal structure, which in turn is connected to the power supply through the negative pole.
To increase the level of mechanical and corrosion resistance, as well as to improve the decorative qualities of parts, the zinc coating is additionally subjected to chromating, cadmium plating or treatment with phosphate compounds.
Electrolytic galvanizing at home
Do-it-yourself galvanizing begins with the selection of materials.
The electrolyte can be a solution of zinc chloride and hydrochloric acid in distilled water. This is the so-called soldering acid, which is most often used at home. Craftsmen etch zinc in battery sulfuric acid and obtain the electrolyte ZnSO4, but this process is dangerous because the reaction releases explosive hydrogen and heat. In no case should there be a precipitate of undissolved salt crystals in the electrolyte. Pure zinc can be bought at a chemical store or radio market, or it can be obtained from salt batteries or fuses preserved from the times of the Soviet Union.
A glass or plastic container can serve as a galvanic bath. Tripods for the anode and cathode are installed in it. The anode is a zinc plate to which the “plus” is connected from the power source. The larger the anode, the more uniform the coating will be on the cathode, the product on which the protective coating will be applied. There can be several anodes; they can be placed around the cathode at the same distance so that its surface is covered with zinc evenly and simultaneously on all sides. The “minus” of the power source is connected to the cathode.
Even when galvanizing is carried out at home, the technology necessarily includes thorough cleaning and degreasing of the part, as well as its activation in an acid solution.
The power source is a car battery with a low-power incandescent lamp or another consumer in the circuit so that the current in the circuit is lower, or a power supply with a constant output voltage. The main thing is that there is no violent boiling of the electrolyte during the galvanizing process.
Galvanization itself occurs when the anodes and cathode are immersed in an electrolyte and the electrical circuit is closed. The longer the process lasts, the thicker the zinc layer is obtained on the product.
With the help of galvanic galvanizing, the protective coating on products becomes precise, uniform and smooth, with a decorative effect. It is used both in industry and at home, despite the fact that wastewater treatment from environmentally hazardous waste is required.
Types of electrolyte
The use of this technology requires compliance with the electrolyte composition and temperature conditions. This is due to the fact that these parameters, at the required current density, have a direct impact on the structure of the applied coating and the rate of zinc deposition.
To obtain the desired decorative effect, coloring and shine-forming components are added to the electrolyte.
The galvanic galvanizing method involves the use of several groups of electrolytes, which differ in the composition of the formulation:
- Weakly acidic and acidic are the simplest compositions, the creation of which uses sulfates, chlorides, borofluorides and their mixtures;
- Zincate and cyanide are alkaline substances that contain sodium cyanide and sodium zincate, which are dissolved in sodium hydroxide;
- Ammonia - neutral and alkaline compounds obtained by dissolving zinc oxide in a mixture of ammonium chloride or sulfate.
Technologists also use electrolytes created on the basis of amino compounds. However, such solutions are used extremely rarely.
Nickel plating
Protection of metal products can be carried out using various technologies. One of the most popular and widespread at the moment is nickel plating. This popularity is explained by the chemical properties of nickel. It has a high degree of resistance to corrosion in an aqueous environment, and nickel oxide prevents subsequent oxidation of the metal. In addition, nickel is poorly affected by salts, acids and alkalis, with the exception of nitric acid. For example, a galvanic coating with a thickness of 0.125 mm reliably protects against most industrial gases, which are characterized by increased aggressiveness. This point is also very important: almost all metals can be plated, so this method can be used for additional processing of products.
The use of nickel plating is appropriate for solving a number of problems:
— ensuring the protection of metal products;
— use as a decorative coating;
— formation of a preliminary layer that will be subjected to further processing;
— restoration of parts and assemblies.
The coating is characterized by increased wear resistance and hardness and is recommended for parts that operate under friction conditions, especially in the absence of any lubrication, is used to protect against corrosion, as well as ensure high-quality soldering of low-temperature solders, all of this is prescribed in GOST. Galvanic coatings are highly brittle, so it is not recommended to flare and bend parts that have undergone nickel plating. It is recommended to use it for complexly profiled parts. After a heat treatment procedure at a temperature of 400 degrees Celsius, the coating acquires maximum hardness.
Methods
There are several methods for galvanizing metal structures. They are selected depending on what technical characteristics of the protective layer need to be obtained. Types of processing:
- Hot galvanized metal. The procedure is carried out in baths filled with molten zinc. Before being immersed in containers, metal structures undergo several stages of preparation. After the preparatory steps, the product is coated with flux and immersed in molten metal. The last stage is cooling.
- Cold galvanizing of metal parts. To carry out this procedure, a special chemical composition Zinconol is used. It is applied with a brush or roller. After application, a durable protective film is formed on the metal surface.
- Thermal diffusion galvanizing. The second name of the method is sherardization. The metal structure is placed in a container and covered with zinc. After this, the internal space is heated to 2600 degrees. Zinc takes on a gaseous state and penetrates into the upper layers of the workpiece.
- Galvanic galvanizing of metal. The surface of the workpiece is exposed to electricity. A protective film 30 microns thick is formed.
- Gas thermal spraying. During processing, a dry zinc mixture is used, which is sprayed onto the surface of a metal sheet in a gas environment.
Gas thermal spraying of zinc
Electrochemical galvanizing
Using this technology for processing small products, it is possible to obtain a coating whose thickness does not exceed 10 - 15 microns. However, this thickness of the protective layer is not sufficient for the parts to be used for external fastening.
Of course, the technique makes it possible to create coatings of greater thickness, but this requires increased electrical energy consumption. It is also worth considering that waste solutions contain complexons, which complicate the process of processing solutions.
Sherardization technology (thermal diffusion galvanizing) allows you to create uniform protective coatings of sufficient thickness (25 - 30 microns), eliminating the possibility of parts sticking together. However, this processing method also involves increased consumption of thermal energy required to heat the equipment, as well as regular repairs of work boxes.
In addition, the relatively low level of use of zinc powder does not allow the use of technology to provide reliable anti-corrosion protection of fastening elements.
Layer thickness
The thickness of the galvanic coating is determined according to data on the average thickness of the applied layer and depends on the conditions in which the part will be used. They are divided into groups:
- Light conditions (LC) - parts are used in closed heated rooms with a relatively dry atmosphere, or the product will be used for a short period of time in an external environment where there are no active corrosive agents. The thickness of a single-layer coating is about 7 microns, a multi-layer coating is 15 microns.
- Average Conditions (AC) - Parts will be used in environments with average humidity, pollution, small amounts of fuel, industrial emissions or seawater vapor. The thickness of a single-layer coating is 15 microns, a multi-layer coating is 30 microns.
- Severe conditions (HS) - involve the operation of parts in conditions of high humidity, increased levels of pollution with industrial gases, fuel waste, solids, and dust. The thickness of a single-layer coating is 30 microns, a multi-layer coating is 45.
Data on the thickness of galvanic coating of parts in one layer is contained in GOST 2249-43. This includes zinc coatings. Controls multilayer application of galvanic coating GOST 3002-45 (nickel coatings). The thickness of the layer can be changed according to design requirements or in cases where the workpiece is designed for a short service life. The service life of galvanizing is up to 5 years, for other types of coatings – up to 3 years.
How to perform the procedure at home?
To perform galvanic galvanizing at home, you need to prepare equipment, consumables, and study the processing technology in theory.
You can assemble the electroplating apparatus yourself. To do this, you need a direct current source, a device for holding the part, and an electrode. The working fluid will be any salt-based solution that contains zinc.
Factors influencing the thickness of the protective layer:
- working solution temperature;
- metal structure shape;
- density of stress influence per unit of processed area;
- electrolyte temperature.
To prepare a high-quality working solution, you need to open the working battery and drain the electrolyte from it. Then dissolve zinc in the electrolytic liquid. Before use, the prepared solution must be filtered.
The zinc-containing composition must be applied in two layers to increase the anti-corrosion properties. Methods for obtaining zinc for preparing a working solution:
- remelting fuses that were manufactured during the Soviet era;
- buy in stores with chemical reagents;
- from salt batteries.
When galvanizing, the working composition should not boil too much. This indicates a large current that needs to be reduced. Over time, a protective layer forms on the surface of the cathode, which will gradually increase. The galvanized part is cooled and cleaned of soldering acid residues.
Galvanic galvanizing is a method of increasing the corrosion resistance of metal. It involves the application of voltage aimed at activating the zinc-containing solution. If desired, you can process metal blanks yourself.
Watch a video about galvanizing at home:
https://youtube.com/watch?v=JQwjn0TQB10
Electrolytes for galvanizing
For galvanic galvanizing, depending on the purpose of the product, electrolytes are used, which are divided into two main groups.
Electrolytes in which zinc is present in the form of simple hydrated ions are called simple acidic. These are hydrofluoride, sulfate and chloride solutions.
Complex complex acidic and alkaline electrolytes contain zinc in complex ions with positive and negative charges. These are ammonia, pyrophosphate, cyanide and other solutions.
The deposition rate and then the quality of zinc deposits on the product (cathode) depend first of all on what electrolyte is used.
Of the complex electrolytes, zinc deposits on the cathode with high ion scattering. As the current density increases, the metal yield decreases and the hydrogen yield increases.
Therefore, galvanizing in complex electrolytes is carried out at low current densities, and the coating is of very high quality, fine-grained and uniform.
In weakly acidic simple electrolytes, galvanic galvanizing, including at home, takes place at a high current density, at a higher speed than when using complex solutions. The appearance of the products is good, but the coating is not of very high quality and is only suitable for products of a fairly simple shape.
Possible defects and their causes
- Chrome does not settle on the workpiece. The reason may be poor contact, a film of oxides, or a small distance between the electrodes. The process is disrupted due to incorrectly selected conductor cross-sections, excess sulfuric acid, low current density or too hot electrolyte.
- The surface shine is absent or uneven (with darkening and spots). The temperature regime of the electrolyte and the concentration of the reagents are not observed. The current has been exceeded.
- There are growths of metallic chromium on the corners of the object. Current density is higher than recommended.
- Defects (sinks) on chrome plating. Poor cleaning. Excess current, hydrogen retention.
- Peeling of coating. Poor degreasing, surges in voltage, current density or temperature.
The process of how to do chrome plating of parts yourself is attractive due to its accessibility and obvious cost savings. You don’t need to have a special education to chrome-plate a set of wheels or an entire body, or get original handles for doors or cabinets.
Decorating plastic with a layer of chrome in the home workshop is no more difficult than metal
The key to a brilliant result will be thorough compliance with safety rules and attention to the details of the technological process.
Galvanization technology
Galvanic coatings are demanding in terms of surface preparation. Before starting work, it is necessary to thoroughly clean and degrease the parts.
For metal surfaces, it is recommended to use organic solvents that do not cause corrosion, for example MODENGY Metal Cleaner
It effectively removes petroleum products, silicone oils, preservative compounds, adsorbed films, gases, moisture and other types of contaminants. Evaporates quickly and without residue.
Galvanic coating highlights all chips, scratches and cavities on surfaces, so the product being processed must be perfectly prepared.
Next, we will consider galvanization technology.
A negative charge is applied to a part immersed in a container of electrolyte, causing it to become a cathode. A free-standing metal plate receives a positive charge and takes on the function of an anode.
It is this plate that serves to form the coating. When the electrical network is closed, the metal from it dissolves in the electrolyte and is directed to the cathode, where it forms a uniform thin film.
This galvanization method is called anodic. Thanks to it, when corrosion occurs, it is the galvanic insulation that is destroyed, and the protected metal remains untouched for a long time.
There is another galvanization method - cathode sputtering. It is used much less frequently. If the integrity of such a coating is damaged, the intensity of destruction of the metal underneath increases. This is facilitated by the application technology itself.
An electrolyte is a conductive solution that allows metals to flow to the cathode from the anode. The size of containers for this liquid may vary and depend on production tasks.
Large parts are suspended in large baths. For smaller products, electroplating is applied in drum tanks, where a negative charge is applied to the drum, which rotates in the electrolyte. To process very small parts (hardware, fasteners), bell-type filling baths are used. During operation, they rotate at low speed, as a result of which the parts are evenly covered with a protective coating.
The current density that passes through the electrolyte is of great importance. It affects the structure of the formed sediment. This value is measured by the ratio of current strength to a unit surface of the workpiece.
If the density is too high, there are a lot of powder deposits, but if it is low, it does not form at all. This affects the quality of the final coating. That is why the galvanization process requires constant monitoring.
Defects during galvanic galvanizing
Among the reasons that significantly affect the quality level of processed parts are the following:
- Low quality of preparation of metal structures;
- Deviation from compliance with the electrolyte recipe;
- Violations of the characteristics and sequence of stages of galvanic processing.
Also, the quality of the finished product depends on the configuration, features of the location and condition of the planes of the leading and additional anodes, as well as the spatial arrangement of the products in the electrolyte.
As a result, the parts may contain defects such as:
- Pitting – deepened stripes or minor pinpoint cavities form on the metal. Such disadvantages appear, as a rule, as a result of the presence of hydroxide or organic impurities in the electrolyte, as well as with low mixing intensity or its complete absence.
- Low level of adhesion - poor adhesion of the zinc layer or its peeling can be observed if the cleaning, etching or degreasing process of the part is violated. This is also observed when the electrolyte is clogged with various organic compounds, including salts of various other metals.
- Variation in appearance causes non-compliance with the recipe in terms of the proportion of electrolyte components used while simultaneously accumulating a certain volume of iron salts in the galvanic bath. Also, the cause of this defect may be insufficient mixing of the components and low temperature, which does not meet the norm.
- Increased roughness indicates the presence in the galvanic mixture of all kinds of mechanical impurities, zinc sulfate and hydroxides in an increased volume. This also occurs as a result of an insufficient amount of zinc anions in the electrolyte and an excessive current density.
- The fragility of the zinc coating is a consequence of excessive current density in the cathode space or the presence of large amounts of organic impurities in the electrolyte.
- Dark (mostly brown) color – causes the presence of various organic contaminants in the galvanic bath. This effect can also cause a significant decrease in the current density near the cathode and an increase in the temperature of the electrolytic mixture.
Purposes of Metal Electroplating
There are a variety of purposes for electroplating. For example, for galvanic chrome plating, the surface must first be coated with nickel. Electroplating is usually used to improve the decorative and protective qualities of structures. This procedure is also used to make exact copies of complex elements. In this case, the process is usually called galvanoplasty.
The technology of galvanizing metals through galvanization is widespread. It allows you to create a zinc coating on the surface, which is characterized by excellent anti-corrosion properties. Products made of metal alloys that have been processed using this technology can retain their properties for a long time at high humidity and even with constant exposure to salt and fresh water. Galvanizing is also used to process pipe products, all kinds of containers, support and building structures. Thanks to the use of galvanizing, metal surfaces receive both electrochemical and barrier protection.
If galvanizing only increases the resistance of the material to corrosion, then galvanizing with chromium solves this problem, making the surface more wear-resistant and stronger and also improving its appearance. Nickel-based galvanic coatings have a similar effect.
Another area of application of electroplating is the jewelry industry. This technology in this situation is used to improve the appearance of jewelry. In this case, a layer of silver or gold is applied to the jewelry. In addition, the film that is applied to the product during processing makes it brighter and more attractive.
Mechanical galvanizing
The technology significantly increases the level of use of zinc powder (almost up to 90 - 95%), and also makes it possible to create coatings with a thickness of 6 to 80 microns (depending on the amount of powder used).
This method does not require large production costs due to the fact that it only requires energy consumption for rotating the drum for a single load of up to 1 ton. In addition, waste recycling is easy, since there are no complexones in the waste solutions.
The standard algorithm for mechanical galvanizing consists of the following activities:
- Cleaning the surface from rust and other contaminants;
- Preparing parts for processing;
- Adding a promoter;
- Using zinc powder.
The cleaned parts are placed in a drum, into which cationized or sodium-ionized water is subsequently added for softening, as well as conditioners necessary to remove residual oxides, various deposits and contaminants. Conditioners also temporarily give the surface a copper color.
Next, zinc powder and glass beads measuring 5, 1.5, 0.7 and 0.25 mm are added to the solution, after which the drum is started, which rotates at an angle of 30° relative to the horizon at a speed of 43 to 75 m/min. Thus, the balls evenly “drive” zinc particles into the metal surface and directly into the zinc coating.
The thickness of the finished coating directly depends on the volume of powder added to the drum, while it is absolutely independent of the duration of processing (as happens during sherardization). Thus, in the same period of time it is possible to create a coating of 15 microns or 75 microns. The processing process usually takes about 45 – 60 minutes.
At the final stage, the processed material is removed from the drum and the process of separating the steel parts from the glass beads begins. The separation process can take place using a magnetic method, vibration forces or a combination of both methods.
In addition to zinc coatings, this technique allows you to create coatings based on tin, copper, cadmium, lead or brass. The result is layer-by-layer or combined coatings from mixtures of different metals.
For example, using a solution consisting of 75% zinc and 25% cadmium can achieve a synergistic effect (the mixed coating layer provides more reliable protection than each of the metal layers separately). The finished coating has a porous structure; it can be coated with a layer of chromium, various lubricants, oils and varnishes to increase the protective properties and the desired color.
The use of this technology makes it possible to give nails and other fasteners greater holding force compared to sherardized or hot-dip galvanized products. Due to the fact that nails do not stick together during mechanical galvanizing, they can be processed in automatic equipment, which involves tying together large areas of wooden frames with nails. Let us recall that not so long ago it was possible to use fastening elements on such equipment, the galvanization of which was carried out by the electromechanical method.
The advantages of mechanical galvanizing technology also include the ability to process products at room temperature. Thus, unlike hot-dip galvanizing technology, which involves treating steels with a strength of no more than 35 Rockwell, this method can be applied to high-strength steels due to the absence of the formation of the brittleness effect.
This makes it possible to galvanize fastening elements for fixing concrete products and other durable structures. Thus, the range of fastening elements can be quite wide, including staples, nuts, stamps, screws, washers, fittings, chains. However, only parts whose length does not exceed 300 mm and whose weight does not exceed 1 - 2 kg can be processed.
Do-it-yourself metal chrome plating technique
Equipment for chrome plating metal is quite easy to make yourself. In most cases, it includes:
- glass or plastic container;
- thermal insulation and sealed lid of the working container;
- heating element with thermostat;
- power supply with a power of 1 kW and a voltage of 10÷12 V;
- lead anode with terminal;
- a device for hanging and a clamp for fastening a part with a terminal;
- tanks for etching and washing, wires, stand and other minor equipment.
The layout of such a chrome plating kit depends on the size and features of the elements included in it and is done “by eye”, with additions and changes during production. It is better to read about current modes in advance in specialized publications or communicate with knowledgeable people on specialized forums. There you can also discuss the issue of the influence of chromium on the properties of steel and other metals, since the mechanical characteristics of a chrome-plated part will change somewhat.
Preparing the surface for chrome plating
Chromium plating of aluminum and its alloys requires a special approach to pre-treatment of the surface of these metals, since they always have a stable oxide film on them. The sequence of their preparation for electroplating looks like this:
- Washing the entire metal surface in gasoline.
- Removing traces of gasoline in hot soapy water.
- Etching in a mixture of nitric and hydrofluoric acids (ratio five to one).
- Rinse in cold water.
- Placing the product in a galvanic bath.
All operations should be performed in continuous sequence, and the metal should be immersed in the electrolyte under current.
Types of coatings
The galvanic coating method is implemented by applying various metals to the product, each of them has its own characteristics and purposes in the further operation of the part or object:
- Silver plating – increases aesthetic value, protects against corrosion, improves reflective and conductive characteristics. This type of application is in demand in the production of static relays, contactors, electromagnetic relays, electromagnetic starters, microcircuits and other electronic products.
- Nickel plating is the most popular galvanic coating of steel, copper and aluminum products. The nickel layer reliably protects products or machine parts from rust formed under the influence of the external environment, as well as from types of corrosion resulting from contamination of the working environment by aggressive media - alkalis, acids, salts. Nickel-plated products demonstrate high resistance to severe mechanical damage and abrasion.
- Chrome plating - increases the wear resistance and hardness of anodized surfaces, improves the appearance, and restores damaged parts to their original parameters. Depending on changes in the technological regime, a galvanic coating with different parameters and properties is obtained - matte gray (increased hardness, but low wear resistance), shiny (high wear resistance, hardness), milky plastic (aesthetics, high degree of anti-corrosion protection, low hardness), galvanizing – anti-corrosion treatment of solid steel sheets, car parts, construction and finishing materials.
- Electroplated gold plating - used in jewelry, electronics and other fields. The gold layer gives the parts high reflective properties, aesthetics, protection against corrosion, and increases conductive qualities.
- Copper plating - often used to coat metal to protect against corrosion; copper improves current-conducting qualities; metal with such a coating is often used for the production of electrical conductors used outdoors.
- Brass plating – used to protect steel, aluminum and alloys from corrosion damage. The brass layer provides the necessary adhesion of metal parts to rubber.
- Rhodium plating is a special coating applied to give parts high resistance in chemically aggressive environments and to obtain additional mechanical wear resistance. Rhodium plating also adds decorative value to products and protects silver items from oxidation and dullness.
Regulation of the quality and technological processes of galvanic coating occurs using GOST 9.301-78.
Methods of applying zinc coating
Anti-corrosion galvanizing is performed in various ways, and the service life of the coating depends on the thickness of the protective layer.
The method of applying the coating depends on its required properties, the size of the product, and the conditions of its further operation.
The simplest and most technologically advanced, but insufficiently providing resistance to mechanical impacts of the protective layer, is cold galvanizing using primers that contain large quantities of highly dispersed zinc powder.
In terms of the volume of galvanizing production, hot galvanizing ranks second. The coating obtained in this way is high-quality and durable, but environmentally unsafe, since molten zinc is used, and maintaining its temperature slightly less than 500 ° C requires a large amount of electricity and chemical surface preparation methods.
Very similar to hot-dip galvanizing, a more technologically advanced, but less productive method of thermal diffusion application of a protective layer. It is used when high demands are placed on the thickness and appearance of the coating.
Another galvanizing method is thermal gas spraying, which is used to protect large-sized products and structures that simply cannot be placed in a bath.
Galvanic galvanizing does not have many of the disadvantages of other coating methods and has its positive aspects.
What kind of electroplating will he do for you?
— zinc coating (zinc plating) – gives products shine and prevents the formation of rust;
— nickel coating (nickel plating) makes the metal part resistant to external influences;
— copper coating (copper plating), which we do upon pre-order, forms a durable protective film for parts;
- plating with gold or silver (gilding and silvering), which is carried out on special orders of sufficient volume, will provide a combination of an extremely expensive appearance and reliable protection against corrosion;
— chrome coating (chrome plating) qualitatively improves the aesthetics of products, while making them more durable and increasing protection from aggressive external environments;
— brass coating (brass plating) gives the products a stylish decorative look;
— etching removes the surface layer from the product, which allows you to remove oxides and rust and detect internal defects. The procedure becomes an excellent preparation for applying the topcoat;
— aluminum electroplating creates a galvanic coating on this difficult-to-process material and solves the difficulties associated with its surface oxide film.
Specialists carry out all the necessary operations, competently selecting the mode of the electrolytic process to suit the conditions of the order.
Three good reasons to outsource your order
Dangerous moments
When using acids as electrolytes, safety precautions must be strictly observed. Neglecting them can lead to accidents:
- In case of contact with skin, due to the fact that the drug is diluted, minor burns are possible. But such a concentration is dangerous for the eyes, so you should not neglect protective glasses and gloves.
- Under the influence of current, oxygen and hydrogen vapors are released, which, when mixed, form an explosive gas. When working in a poorly ventilated area, you can get an explosion from any spark, which can be fatal.
https://youtube.com/watch?v=DYxNFTKW1BY
By following safety precautions and the stages of technological processing, you can get durable, beautiful things: chrome plating car wheels, creating gold-look jewelry, adding strength to parts of household mechanisms, depending on the technologies used.
In production, galvanic processing of metal is carried out in a strict sequence
- Cleaning the surface of parts from paints and lubricants, rust and scale (the procedure is carried out using degreasing and alkaline mixtures).
- Rinsing with clean water in a special flow bath.
- Electrolytic degreasing and subsequent rinsing.
- Etching in a composition that includes water and hydrochloric acid. The procedure removes rust and scale residues, eliminating dissolution or deformation of the base metal, and also picks up surfaces before processing.
- Washing, direct galvanizing and re-rinsing.
- To remove the oxide film from the surface, use metal brightening in a solution consisting of water and nitric acid.
- Washing, phosphating (if necessary) followed by washing.
- Passivation can be carried out by electrolytic chromating or by chromating spraying.
- Drying parts.
Depending on the features of the processing technology and the type of product, galvanic processing of metal may include additional manipulations.
If a strip is processed, galvanizing begins with unwinding the material, and then welding the ends. At the final stage, the strip is treated with oil and rolled up.
Materials and equipment
Galvanic processing of various materials involves the use of appropriate “consumables” and equipment. To coat elements with metals, the same type of galvanic installations are used. The difference will only be in the composition of the electrolyte solution used, its temperature and operating modes.
So, the procedure can be performed using the following equipment:
- special baths with electrolyte in which the element being processed and anodes are placed;
- an electric current source that is equipped with an input voltage regulator;
- a heating device that will bring the electrolyte to the desired temperature.
Anode plates are also required, which will supply voltage to the electrolyte and distribute it throughout the element being processed.
It should be noted that hazardous compounds are used to make electrolytes, so they must be stored in safe containers.
Any galvanic equipment must be located in areas with good ventilation. You also need to be very careful about safety requirements. All activities associated with galvanic processing must be carried out wearing a protective respirator and goggles, as well as special shoes, an apron and gloves. If electroplating is carried out at home, then you should first study the relevant literature or watch video tutorials on this topic.
Chemical method
This method is considered expensive relative to the electrolytic method. The result is a fairly strong and thin base of the applied layer.
Nickel plating of parts is carried out as follows:
- Take a 10% solution of zinc chloride and dilute it in small portions in a solution of nickel sulfate until a bright green tint is obtained.
- Next, using a porcelain vessel, the resulting mixture should be heated until boiling. There is no need to be afraid that the result will be muddy; this will in no way affect the quality of the planned work.
- For nickel plating, you should lower the part, previously cleaned of dust and degreased with soda solution, into a boiling solution.
- The boiling process should last at least an hour, but as the liquid evaporates, distilled water must be gradually added to the container. If the rich green color becomes lighter, this means that it is necessary to add a small portion of nickel sulfate.
- After the boiling time has passed, remove the part and rinse it in water with chalk dissolved in it.
- Dry thoroughly in the open air.
Products made of ferrous metal coated with this method are durable and reliable during operation.
Analysis of the chemical application of the protective layer shows that the ongoing process underlies the recovery of nickel from salt liquid using sodium hypophosphite and other elements. Solutions can be either alkaline or acidic.
The purpose of acid compositions is better suited for processing non-ferrous or ferrous metals. Alkalis are intended for application to stainless steel surfaces.
The acid provokes a decrease in discharge with increasing temperature, but the surface is obtained with a lower roughness index. When using this composition, good adhesion of the coating to the surface is ensured.
A water-based solution for nickel plating, used for all metals. You can use not only distilled water, but also condensation formed in the refrigerator. It is better to use clean chemicals with the letter “C” on the packaging.
To obtain a solution, initially all ingredients are diluted in water, and then sodium hypophosphite is added. One liter of solution is enough for nickel plating of a surface area of 10x10 cm2.
Galvanizing small parts – which method to choose?
Metal parts are used in all structures and equipment, be it industrial machines, complex machines, or simple utility carts. Even ordinary bolts, nuts and screws are made of metal. No one wants a working machine or industrial machine to stop working because of one failed bolt. But sometimes this is exactly what happens, since every metal part, even the smallest one, suffers from corrosion.
The metal itself has a strong structure, but this does not save it from the harmful effects of the environment. To preserve the performance of the metal, it is simply necessary to use anti-corrosion materials.
Galvanizing has long been recognized as the most effective way to combat corrosion. Galvanizing is the coating of metal with a thin layer of zinc. Coating is applied in various ways; there are: hot galvanizing, galvanic, thermal diffusion, cold galvanizing and other methods of coating. In this article we will tell you which method is best for galvanizing and protecting small parts from corrosion.
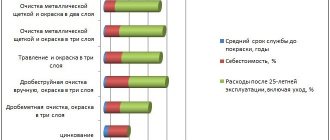
Various galvanizing methods
Each galvanizing method has its own characteristics, which can be both pros and cons in each specific case. When choosing a method, they choose the one that is more convenient, economical and better suited for further operating conditions. Sometimes, several galvanizing methods are used in the same design or product. But it is worth noting one common proven feature - the coating lasts longer and more reliably, the more and purer the zinc in its composition.
Hot galvanizing of small parts
Hot galvanizing of small parts is a process that protects their surface from corrosion. It involves immersing metal parts with a pre-cleaned surface (chemical cleaning) in a bath of hot zinc (the temperature is maintained within 450-460 ° C), which, reacting with iron, forms a thin protective layer of galvanization on the surface of the products.
- Advantages.
The resulting zinc coating has unique properties: it does not interact with petroleum products, various solvents, lubricants and other commonly encountered chemically active elements of the environment. A galvanized product, as a rule, has a coating thickness of 35 to 120 microns. If necessary, you can achieve a significant increase in the thickness of the protective layer (up to 180 - 200 microns). Hot-dip galvanizing is used for small parts made from any mild steel, low-alloy steel, and cast iron. - Flaws.
Hot-dip galvanizing will cost you little, only for large volumes of small parts, since the cost is mainly calculated based on a ton of iron. The method is not suitable for some types of iron and steel, and is also not used for coating threaded parts and parts with narrow holes, due to the unevenness of the zinc layer, which can block the holes and make the parts unusable. Due to the use of high temperatures (over 400°C), thin parts may become deformed.
Galvanic zinc plating of small parts
During galvanizing, parts are immersed in a special electrolytic solution. A cathode from a direct current source is connected to the parts, and a zinc bar or sheet of zinc connected to the anode is lowered into the solution. The metal ions in the electrolyte begin to move from the zinc metal to the part being coated, where they settle in a thin layer.
- Advantages.
In this way, you can simultaneously coat a large number of parts of various shapes and sizes with an even layer of zinc. Another advantage is low cost and high speed. - Flaws.
The disadvantages include weak adhesion between the metal and the coating, as a result of which the products are poorly resistant to physical stress and significant deformation. Therefore, galvanic processing is used to galvanize pipes, sheet iron, metal structures, fasteners and, much less frequently, small parts. If cyanide electrolyte is used, there is a danger to the environment.
Thermal diffusion galvanizing of small parts
The essence of thermal diffusion galvanizing is that, under the influence of high temperature, powdered zinc particles interact with iron, resulting in sintering of the two components with the formation of an intermediate diffuse layer in which the interpenetration of iron and zinc into each other occurs. The result is a reliable protective layer that perfectly resists corrosion, mechanical stress and the penetration of aggressive substances into the product.
- Advantages.
The diffusion coating makes up up to a third of the total layer thickness and ensures good adhesion of materials. The diffusion layer covers the entire surface of the product, even threaded connections, small structural elements and markings. The treated surface has a high class of cleanliness. Due to the fact that there is no need for preliminary acid pickling, the metal product does not lose its mechanical properties, whereas with other galvanizing methods, parts often become brittle. Due to the fact that the entire process takes place in closed containers, the method is highly environmentally friendly and has no harmful emissions. - Flaws.
The treated surface does not have a shiny decorative appearance, but since it is intended primarily for industrial enterprises, this drawback can be neglected. The processed parts have porosity and uneven coating. When carrying out processing, you must carefully monitor compliance with all safety rules and the tightness of the system, since zinc dust involved in the technological process is dangerous to human health. The need to use a large amount of equipment: an electric furnace with a vertical loading chamber, a stainless steel cylinder, a special mechanism for lowering, raising and rotating the cylinder. A large number of complex equipment and high requirements for premises make the method little widespread and therefore inaccessible and expensive.
Several more ways to protect metal
Metallization method
based on the adhesion of sprayed metal particles. Spraying of zinc is carried out using a spray gun. The advantage of the method is the possibility of coating large assembled structures with zinc, as well as coating plastics, plaster, wood and other materials. The disadvantage of this method is the increased porosity of the coating and large (up to 50%) metal losses.
Contact method
Zinc deposition is galvanizing without an external current source due to the work of a galvanic couple formed when steel parts are immersed in contact with aluminum in a solution of zinc salt. In this case, zinc is replaced by aluminum. Coatings obtained by this method are characterized by insignificant thickness, low protective properties and are used only for processing non-critical parts.
Electrolytic galvanizing
allows you to achieve: a high degree of purity of deposited zinc and high chemical resistance of coatings, the ability to regulate the thickness of deposited zinc (low metal consumption), good mechanical properties of the zinc coating (elasticity and adhesion of the coating to the base metal). Electrolytic galvanizing is carried out in acidic and alkaline electrolytes.
These galvanizing methods are much less common and are rarely used.
Cold galvanizing of small parts
Cold galvanizing is the application of a coating with a high (more than 92%) zinc content to a cleaned metal surface using a paint and varnish method.
- Advantages
. Quick drying in just 20 minutes. Possibility of application over the coating of almost any paintwork material. High protective characteristics against damage and aggressive atmosphere. Long lasting - up to 50 years without renewal. Possibility of welding parts after coating without destroying it. No corrosion even when damaged. Ease of restoration of damaged coating. Easy to apply - just like regular paint and right on site, which means no transportation costs. - The disadvantages
of this method are strict adherence to the technological process and careful preparation of the surface. But, such points are inherent in all of the above application methods.
One of the main advantages of cold galvanizing when used on small parts is the different application possibilities. That is, in order to treat all hard-to-reach areas of small parts, you can apply the coating by immersion in the composition. If the structure has a complex shape and unpainted areas remain during immersion, they can always be repainted using a regular brush of any size. You can apply cold galvanizing yourself, without resorting to the services of professionals, or right on site. It’s not at all difficult to do this and at the same time save money on packaging and transportation.
Having assessed all the advantages and disadvantages of galvanizing methods, you can choose the most optimal one for you. We recommend that you use cold galvanizing due to objective reasons - more advantages and the virtual absence of disadvantages.
In our store you can choose and purchase a composition for cold galvanizing and protective anti-corrosion enamel, and our managers will help you with your choice and purchase
Have questions about choosing a composition? Contact the representative office in your city:
in St. Petersburg: +7, +7 (921) 927-58-47
in other cities:
8
e-mail:
[email protected]
Automated line for galvanizing
A modern galvanizing line is a fully automated line on which all stages of coating are carried out, including welding and high-quality degreasing of products for various purposes and configurations.
In general, an automatic line consists of a set of technological galvanic baths, modular rectifiers, a loading/unloading stand, transport equipment, equipment for exhaust ventilation, water supply and sewage disposal, a metal frame with a service ladder.
Galvanic baths can be made of stainless steel, steel lined with polymer materials or rubber. Modern bathtubs welded from sheet polymers are increasingly replacing metal containers. The choice of bath material depends on the composition and concentration of the electrolyte and the operating temperature.
Communications of water supply and sewerage systems, and in most cases ventilation are located under the bathtubs and are also made of polypropylene.
The dimensions of the line are determined by its productivity and the dimensions of the galvanic baths.
Galvanic galvanizing occurs with the formation of wastewater with a high concentration of heavy metal ions. Therefore, they are settled, filtered, neutralized, chemical precipitation, sorption and other processes are used in containers made of engineering polymers.
Features of electroforming
Using this method, objects are copied down to the smallest detail. Electroplating allows the production of products with complex configurations that cannot be reproduced in any other way. Fragile workpieces gain strength and a different appearance when coated with metal. The material of the workpiece does not matter, since it remains inside. The copy is as close as possible to the original.
Acorn pendant made by electroplating
The use of both types of electroplating at home allows for the production of decorative jewelry, protective coatings and simply beautiful things. A correctly selected electrolyte and a well-organized electrochemical process is all that is needed for this.
Workshop safety
The harmfulness of the galvanic shop to human health is quite high. The thing is that there are several very dangerous factors. Firstly, there is the possibility of receiving a strong electric shock, secondly, there is a risk of getting chemical, alkaline or acid types of burns, and thirdly, there is a risk of explosion and fire.
However, the harm to human health does not end there. For example, when preparing a product, it is subjected to mechanical types of processing. This can be grinding, blast cleaning using mechanical dust and many others. All of them are united by the fact that during their implementation a huge amount of dust is released into the air. In addition, the noise and vibration levels exceed permissible limits. Since electric current is used during coating, the likelihood of injury from this same current greatly increases. For this reason, direct current with a voltage of 12 V is most often used. However, there are some operations that require increasing the voltage to 120 V. For example, this occurs when it is necessary to oxidize aluminum.
Fire safety requirements for electroplating shops are also quite high. To prevent fire in such premises, it is necessary to use fire prevention and fire protection systems that will comply with GOST 12.1.004-76. Explosion safety in such areas must be ensured using explosion prevention and explosion protection measures in accordance with GOST 12.1.010-76.
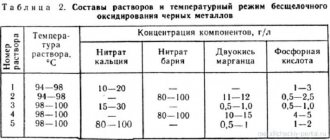
Weak acid or alkaline galvanizing.
It’s very difficult to write about something you don’t really understand))))
And so, we have introductory ones:
baths 1500*600*900 (about the same)
two manufacturers have 10 and 12 baths, respectively: degreasing-rinsing-pickling-rinsing-zinc-plating-rinsing-chromate-rinsing-drying. The meaning of additional baths was agreed upon, but now I don’t really remember. In a line with 12 baths, there is an additional one for galvanizing in order to serve the second one.
Some bathtubs include metal structures, others honestly say, do it yourself, there is no point in selling metal for 3-4 times the price, this is about a simple hoist.
If you make an auto operator, then the cost for some immediately jumps by 8 million rubles for 2 pieces, automation is at the level of a STM controller and some writer in C++, why not on a PLC is not clear, but after laughing I refused, we will make the auto operators ourselves and with with their algorithms. By the way, private labels from both manufacturers.
Some promise weakly acidic galvanizing and promise the desired shine (samples from slightly acidic galvanizing). At the same time, expensive additives are needed, while up to 60% of the water can be returned back into the process with the help of treatment plants. They promise to adjust the process once a week at our volumes. There are simple on-site tests to determine the quality of the solution. But the environmental friendliness of this process, as others say, is low, even taking into account the treatment facilities.
The latter offer alkaline, which is essentially called soap, but the process is more demanding on the solution (as far as I understand) and requires more frequent adjustments. In this case, you can return less water to the process, but the cleaning is simpler, that is, more environmentally friendly.
In both cases, treatment facilities come separately and for quite a lot of money, but since we are for the environment, we will definitely take it. The main question was how suitable treatment plants alone are for both processes. The output from both is salt water (as far as I understand) and sludge, which falls under disposal class 4.
The baths themselves are made using approximately the same process: plastic-CNC gluing (the gluing is different, but on average it’s the same garbage), overflow in the baths, filters, bubblers, and other goodies for galvanizing.
Some offer ventilation as part of the composition, others for extra money. The differences in the main process are minimal, the price differs significantly...
That's pretty much it.
So the question is, can the guys who offer the alkaline process make normal cleaning for a slightly acidic one? Or does it depend little on them? and will those who mainly use alkaline be able to start the slightly acidic process normally and explain how this is done without them. It is clear that zinc generators are needed. Both power sources are Russian.
Electroplating at home
This process requires not only certain knowledge in the field of chemistry and physics, but also equipment that you can make yourself.
To do this you need:
- find a power source, which can be a rectifier, a charger from an old mobile phone or another device with low power;
- copper wires of two types - thick and thin, through which the electric current necessary during the entire electroplating procedure will pass;
- a sufficiently deep container or bath made of glass or plastic, and it must also be durable to withstand high temperatures (up to 80 degrees);
- anodes with a larger area than the parts being placed to ensure uniform current distribution and for the normal occurrence of some oxidation processes;
- devices for heating the electrolyte, such as an iron or a small electric stove.
Technological features of the process
The technological feature and complexity of galvanic chrome plating, copper plating, silver plating or other coating lies in a multi-stage process. At the initial stage, it is necessary to prepare a medium from the above materials and prepare an electrolyte. To prepare it, you need chemical reagents measured in certain proportions accurate to the nearest gram. Of course, to achieve such high accuracy, you need special scales (preferably electronic).
Then you can proceed to the next stage: the prepared electrolyte is poured into a container, the anodes are lowered into it and connected to “+”, and a product is placed between them, which is connected to “-“, thus the circuit is closed, and the released metal in the electrolyte is deposited on the surface products.
Product preparation
Before you begin coating the product, you must thoroughly clean its surface.
This is very important, since the quality and durability of the coating will depend on this stage. For these purposes, the product goes through several stages of cleaning: from degreasing to grinding and sandblasting
To degrease the product, any organic substances can be used, for example, acetone, thinner, gasoline or alcohol. However, the solution may be different depending on the material of the product.
Recently, galvanic baths have become widespread; to give a decorative effect, cast iron and steel baths began to be coated with copper and nickel. Therefore, to degrease products made from such materials, special hot solutions of caustic soda, liquid glass, sodium oxidized with phosphoric acid, or soda ash are used. Or, if the product is made of non-ferrous metal, use a solution with laundry soap. Thus, the product is degreased, cleaned and polished. And then they are lowered into a container with electrolyte and anodes, where the coating is applied to the surface.
Scope of application of galvanic galvanizing
This method is widely used on products made from carbon steels and various types of cast iron. The main range of electroplating is represented by various tools, machine and equipment parts, all kinds of supports and fasteners, including thin-sheet cold-rolled metal.
Along with protective properties, galvanizing also gives the metal decorative qualities. This is due to the uniform distribution of the coating over the surface and the exact repetition of the coating of the part configuration.
The thickness of the zinc coating is 6–9 micrometers, but the structures are passivated in a special chromate solution. Thanks to passivation, a high aesthetic effect can be achieved.
The procedure allows you to give structures such colors as rainbow (golden color that shimmers perfectly in the sun) and blue (white zinc takes on a blue tint).
The electroplating technique involves only the external coating of parts, since it is impossible to apply coating in hard-to-reach places due to the lack of electrical conductivity.
Metal structures, galvanized by galvanic method, are widely used in temperate environments. Thus, such structures can be used outdoors only periodically, and they should not have direct contact with moisture.