Acetylene production
Acetylene can be produced in laboratory and industrial conditions. To obtain acetylene in the laboratory, it is enough to drop a small amount of water onto calcium carbide (this is its formula - CaC2). after this, a violent reaction of acetylene release begins. To slow it down, it is permissible to use table salt (NaCl formula).
In an industrial environment, things are somewhat more complicated. To produce acetylene, pyrolysis of methane, as well as propane and butane, is used. In the latter case, the acetylene formula will contain a large number of impurities.
The carbide method of acetylene production ensures the production of clean gas. But, this method of obtaining a product must be provided with a large amount of electricity.
Pyrolysis does not require a large amount of electricity, the whole point is that to produce gas, it is necessary to heat the reactor and for this they use gas circulating in the primary circuit of the reactor. But in the flow that moves there, the gas concentration is quite small.
Isolating acetylene with a pure formula in the second case is not the easiest task and its solution is quite expensive. There are several ways to produce the acetylene formula in an industrial setting.
Electric cracking
The conversion of methane into acetylene occurs in an electric arc furnace, where it is heated to a temperature of 2000-3000 °C. In this case, the voltage on the electrodes reaches 1 kV. Methane is heated to 1600 °C. To obtain one ton of acetylene, it is necessary to spend 13,000 kWh. This is a significant disadvantage of producing acetylene formula.
Technological diagram of cracking
Oxidative pyrolysis
This method is based on mixing methane and oxygen. After producing the mixture, part of it is sent for combustion and the resulting heat is sent to heat the raw materials to a temperature of 16,000 °C. This process is characterized by continuity and rather modest consumption of electrical energy. Today, this method can most often be found in acetylene production plants.
Technological diagram of the oxidative pyrolysis process
In addition to the listed technologies for the production of acetylene formulas, such as homogeneous pyrolysis and low-temperature plasma are used. All of them differ in the amount of energy costs and, as a result, different characteristics of the resulting gas and its formula.
Acetylene production
When producing acetylene, it is necessary to take into account its high potential for explosive decomposition. The decomposition occurs exothermically according to the equation
С2Н2→2С+ Н2
and is determined primarily by temperature and pressure. With increasing temperature, the explosiveness of acetylene increases sharply.
Gaseous impurities that form flammable mixtures with acetylene increase the ability of acetylene to explode. Such impurities include air, oxygen, hydrogen phosphide, etc. Mixtures of acetylene with air, oxygen and hydrogen phosphide, even if their content is insignificant, explode at atmospheric pressure if the temperature at any point in the mixture reaches the ignition temperature. For acetylene-air mixtures (2.2-81% acetylene), the ignition temperature is in the range of 305-407 °C; acetylene-oxygen (2.8-93% acetylene) 197-306 C; acetylene with hydrogen phosphide 100-200 °C. The content of gaseous impurities that contribute to the explosive decomposition of acetylene is reduced during its production to the minimum possible limits: air to 0.5-1.5%, hydrogen phosphide to 0.08%, hydrogen sulfide to 0.08-1.5%.
Gaseous impurities that do not enter into chemical reactions with acetylene reduce its ability to explode. These include nitrogen, carbon monoxide, methane, water vapor, etc. This is explained by the dissociation of acetylene molecules by molecules of gaseous impurities.
A similar effect has the dissolution of acetylene in liquids. The highest solubility of acetylene among available liquids is in acetone.
The limit for the explosive decomposition of acetylene is reduced in the presence of catalysts - oxides of copper, iron and other compounds. Therefore, the walls of the equipment during the production of acetylene and its consumption should not have oxides.
acetylene can react with copper and some other metals to form explosive compounds - acetylenides. Their presence will lead to the explosive decomposition of acetylene. Therefore, it is prohibited to use alloys containing more than 70% Cu in equipment for acetylene.
Acetylene is produced from calcium carbide and water in special devices called acetylene generators.
The exothermic reaction proceeds according to the equation CaC2 + 2H20 = C2H2 + Ca(OH)2 + Q.
Theoretically, the yield of acetylene from 1 kg of calcium carbide is 372.5 l (at 20 °C and 0.1 MPa). The actual yield of acetylene is much lower and, depending on the type of carbide, ranges from 235 to 285 l/kg.
Various generator designs are used to produce acetylene. Their typing and classification is based on the following characteristics: productivity, installation method, pressure of acetylene produced, system of regulation and interaction of calcium carbide with water.
Based on the installation method, generators are divided into mobile and stationary. The productivity of mobile generators should not exceed 5 m3/h.
Based on the pressure of acetylene produced, generators are divided into three groups: low (up to 0.01 MPa inclusive), medium (over 0.01-0.15 MPa) and high (over 0.15 MPa) pressure.
According to the systems of regulation and interaction of calcium carbide with water, generators with quantitative control of reacting substances and time-based ones are distinguished. Quantitative regulation of acetylene is carried out by periodic dosing of either calcium carbide with a constant volume of water in the reaction zone (the “carbide to water” system), or by the dosage of water when loading all the calcium carbide (the “water to carbide” system). A combined system of generators with a dosage of both reacting substances—calcium carbide and water—is also widely used. Time-based regulation of the amount of acetylene in the gas collector is carried out by periodically dosing the time of contact of calcium carbide with water. Such generator systems are called “contact”. If the mobile component is calcium carbide, then such a system is called “immersion”; if the mobile system is water, then “displacement”.
There are also generator systems that combine quantitative and time-based control systems (Fig. 25.1).
Rice. 25.1. Scheme of a combined type acetylene generator: 1 - charger; 2 — gas collector 3 — water tank; 4 - gas selection
Acetylene generators, regardless of the system, have the following main elements (Fig. 25.1): charger 7, gas collector 2, safety devices against increasing pressure in the gas collector and protecting the generator from backfire strikes.
These units can be concentrated in one structure or separated and interconnected by pipelines. Stationary generators are in some cases equipped with chemical cleaners.
The charger is designed to load calcium carbide into the generator. In generators of the “water to carbide” system, contact and combined, calcium carbide reacts with water in chargers to form acetylene. Therefore, they are often called gasifiers. In generators of “carbide into water” systems, gas formation occurs outside the charger. In this case, the charger has devices for dosing calcium carbide supplied to the water. Chargers in which gas formation occurs must be well cooled with water and be convenient for removing limescale and washing.
The gas collector is designed to collect acetylene coming from the gas generator and take it to the point of consumption. The presence of a gas collector allows you to compensate for the discrepancy between the acetylene output and its consumption, and also reduce pressure fluctuations due to uneven gas consumption. In the designs of acetylene generators, there are three types of gas collectors: with a floating bell, in the form of communicating vessels and of constant volume.
Safety devices in acetylene generators are used of two types: to release acetylene into the atmosphere when the pressure increases above the permissible level and to protect the generator from flame penetration into the gas collector during a backfire. Backfire is the penetration of the flame front into the burner nozzle channel and its propagation towards the flow of the hot mixture.
The possibility of a kickback is determined by the ratio of the flow rate of the mixture and the rate of its ignition. Backlash occurs when the burner is overheated, the distance of the mouthpiece from the heating surface is small, when the mouthpiece is clogged, etc. Safety devices against an increase in acetylene pressure depend on the design of the generator's gas collector.
Safety devices to protect the generator from backfire strikes are water seals (Fig. 25.2, a, b). The valve body 3 is filled with water to the level of the control valve KK. Acetylene is supplied through tube 7 and passes through check valve 2 located in the lower part of the body. Gas passes into the upper part of the housing through reflector 4. Acetylene is discharged to the point of consumption through the R/S supply valve. In the upper part of the housing there is a tube covered with a membrane 5 made of aluminum foil. When the flame hits back, the membrane ruptures and the explosive mixture comes out. The explosion pressure is transmitted through the water to valve 2, which closes the gas supply from the generator.
Rice. 25.2. Diagram of a closed water shutter: a - normal operation of the shutter; b - reverse impact in the bolt; c - diagram of a dry shutter (flame arrester)
Recently, dry universal valves of the ZSU-1 type have been used to protect against backfire (Fig. 25.2, e). The shutter consists of two independent blocks: flame extinguishing and valve B, installed inside the first using a threaded connection and an O-ring. The presence of two blocks makes it easy to disassemble the shutter and carry out repair work. The flame extinguishing unit consists of an outer housing 1, a cover 4 and a flame arrester 3 and a flame extinguishing element 2 enclosed between them.
After checking the seals 5, the body and cover are sealed. The valve block consists of a housing 6, in which shut-off valves 8 and check valves 7 are installed. Through the inlet fitting, hot gas enters the cavity of the valve block through the open shut-off valve. Next, the gas enters block A through the check valve. Gas is taken out through the outlet fitting. If a backlash occurs, the shut-off valve closes and the gas supply stops. The burning mixture is extinguished in the porous channels of the flame arrester. After the reverse shock is eliminated, the spring returns the valve to its original position.
Chemical cleaners are designed to clean acetylene. Harmful impurities in acetylene (hydrogen sulfide and hydrogen phosphide), passing through the porous mass (gerotol), are oxidized and converted into non-volatile compounds. Chromium compounds are usually used as an oxidizing agent in Gerotol.
Welding stations are powered with acetylene in the following ways: directly at the workplace from mobile acetylene generators or acetylene cylinders; centrally via gas pipelines from cylinder stations.
The quality of acetylene in mobile generators is usually low. Therefore, for direct supply of gas welding stations, it is most advisable to use bottled acetylene.
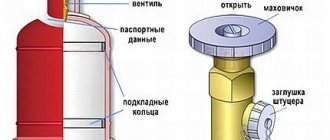
For acetylene, standard cylinders with a capacity of 40 liters are used. The cylinders are filled with a pre-porous mass (activated carbon with a grain size of 1-3.5 mm) and filled with acetone. When filling, acetylene dissolves in acetone and is separated in the capillaries of the porous mass. This filling method eliminates the possibility of explosive decomposition of acetylene even under the most unfavorable conditions. In 1 liter of acetone, 23 liters of acetylene gas dissolves at atmospheric pressure. At a pressure of 1.9 MPa and a porosity of the mass of 70% and a cylinder with a capacity of 40 liters, about 6 m3 of acetylene dissolves.
Centralized acetylene supply is usually used for ten gas welding stations. For a small number of posts, acetylene cylinder ramps are used. The standard range includes ramps consisting of 2×6, 2×9 and 2×15 cylinders. At large and medium-sized machine-building enterprises, centralized power supply of posts is carried out from factory acetylene stations.
The industry produces automated acetylene stations of various capacities, allowing the production of both gaseous and dissolved acetylene.
Advantages
The mention of gas welding immediately brings to mind acetylene. Indeed, this gas is most often used for this process. It, in combination with oxygen, provides the highest combustion temperature of the flame. But in recent years, due to the development of various types of welding, the use of this type of metal joining has decreased somewhat. Moreover, in some industries there has been a complete abandonment of the use of these technologies. But for certain types of repair work it still remains indispensable.
The use of acetylene allows you to obtain the following advantages:
- maximum flame temperature;
- there is the possibility of generating acetylene directly at the workplace or purchasing it in special containers;
- Quite low cost compared to other flammable gases.
However, acetylene also has certain disadvantages that limit its use. The most important thing is the danger of explosion. When working with this gas, it is necessary to strictly observe safety precautions. In particular, work should be carried out in a well-ventilated area. If operating conditions are violated, some defects may appear, for example, burnouts.
Acetylene: application in construction and industry
Autogenous and welding works accompany almost all stages of construction. It is in these types of work that acetylene is used. In a special device called a burner, gases are mixed and the combustion reaction itself occurs. The highest temperature of this reaction is achieved when the acetylene content is 45% of the total volume of the cylinder.
Cylinders with this gas are marked as follows: they are painted white and the inscription “Acetylene” is written in large red letters.
Construction work is carried out mainly outdoors. The use of acetylene and its homologues under these conditions should not be exposed to direct sunlight. Short breaks should be accompanied by closing the valves on the burner, and long breaks should be accompanied by closing the valves on the cylinders themselves.
Acetylene is in great demand in the chemical industry. Its application lies in the use of this substance in the process of obtaining organic synthesis products. These are synthetic rubber, plastics, solvents, acetic acid, etc.
Acetylene, being a universal fuel, is often used in processes involving gas-flame processing. It is important that the use of acetylene in industry is possible only if safety measures are observed, since it is an explosive gas.
Acetylene formula
The structure of the acetylene molecule
Acetylene has a simple formula - C2H2. The relatively cheap method of producing it by mixing water and calcium carbide has made it the most widely used gas for joining metals. The temperature at which a mixture of oxygen and acetylene burns causes solid carbon particles to be released.
Acetylene can be delivered to the work site in special containers (gas cylinders), or it can be obtained directly at the workplace using a specially designed reactor. Where the mixing of water and calcium carbide occurs.
Chemical and physical properties
Some chemical properties
The properties of acetylene are largely determined by its formula. That is, the presence of carbon and hydrogen atoms connected to each other.
Mixing acetylene with water, with the addition of catalysts such as mercury salts, leads to the production of acetaldehyde. The triple bond of the atoms contained in the acetylene molecule leads to the fact that during combustion it releases 14,000 kcal/cu. m. During the combustion process, the temperature rises to 3000 °C.
This gas, under certain conditions, can turn into benzene. To do this, you need to heat it to 4000 °C and add graphite.
The hydrogen contained in the molecules shows acidic properties. That is, they are quite easily separated from the molecule in the form of protons. Acetylene is able to decolorize water containing bromine and a solution of potassium permanganate.
The molar mass of acetylene is 26.04 g/mol. Acetylene density is 1.1 kg/m³.
Physical properties
Under standard conditions, acetylene is a colorless gas that is practically insoluble in water. It begins to boil at -830 °C. When compressed, it begins to decompose, releasing a large amount of energy. Therefore, steel cylinders capable of storing gas under high pressure are used to store it.
This gas must not be released into the atmosphere. Its formula may have a negative impact on the environment.
Acetylene homologues
Acetylene is the simplest representative of alkynes, the molecules of which also contain a triple bond. Propyne CH3C≡CH is a homologue of acetylene. The formula of the third representative of alkynes, butine-1, is CH3CH2C≡CH. Acetylene is a common name for ethylene. The systematic nomenclature of alkynes follows IUPAC rules:
- in linear molecules, the name of the main chain is indicated, which originates from the Greek numeral, to which the suffix -ine and the number of the atom at the triple bond are added, for example, ethyne, propyne, butine-1;
- numbering of the main chain of atoms begins from the end of the molecule closest to the triple bond;
- for branched hydrocarbons, the name of the side branch comes first, followed by the name of the main chain of atoms with the suffix -in.
- the final part of the name is a number indicating the location of the triple bond in the molecule, for example, butine-2.
Welding technology and modes
Acetylene-oxygen mixtures are used to join parts made of carbon and low-alloy steels. For example, this method is widely used to create permanent pipeline connections. For example, pipes with a diameter of 159 mm with a wall thickness of no more than 8 mm. But there are also some restrictions; joining steel grades 12×2M1, 12×2MFSR using this method is unacceptable.
Welding with acetylene
Flame in acetylene welding
Selecting mode parameters
To prepare the mixture necessary for combining metals, use the formula 1/1,2. When processing workpieces made of alloy steels, the welder must monitor the state of the flame. In particular, an excess of acetylene should not be allowed.
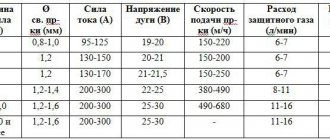
The consumption of the mixture with the oxygen/acetylene formula is 100-130 dm3/hour per 1 mm of thickness. The flame power is regulated using a burner, which is selected depending on the material used, its characteristics, thickness, etc.
To perform welding with acetylene, welding wire is used. Its grade must correspond to the steel grade of the parts being welded. The diameter of the wire is determined depending on the thickness of the metal being welded.
For the convenience of technologists and welders themselves, there are many tables on the basis of which you can quite easily select a welding mode. To do this you need to know the following parameters:
- wall thickness of welded workpieces;
- type of welding - left, right;
Based on this, you can determine the diameter of the filler wire and select the acetylene consumption. For example, the thickness is 5-6 mm, tip No. 4 will be used to perform the work. That is, based on the tabular data, the wire diameter will be 3.5 mm for left welding, 3.5 mm for right welding. Acetylene consumption in this case will be for left welding method 60-780 dm3/hour, with the right 650-750 dm3/hour.
Welding is performed in small sections of 10-15 mm. The work is performed in the following sequence. At the first stage, the edges are melted. After this, the root suture is applied. Once the root formation is complete, welding can continue. If the thickness of the workpieces is 4 mm, then welding can be performed in one layer. If the thickness exceeds the specified one, then a second one must be applied. It is laid only after the root of the seam has been completed along the entire specified length.
To improve the quality of welding, preheating is allowed. That is, the future welded joint is heated using a torch. If this method is adopted as a basis, then warming up must be done again after each stop.
Seams can be made with gas in any spatial position. For example, when making a vertical seam there are some peculiarities. So, the vertical seam should be made from bottom to top.
When performing welding work, breaks in work are unacceptable, at least until the entire seam is cut. When stopping operation, the burner must be withdrawn slowly, otherwise seam defects - cavities and pores - may occur. An interesting feature exists when welding pipelines; a draft is not allowed in it and therefore the ends of the pipes must be plugged.
Types of acetylene
The industry produces two types of acetylene - solid and gas.
Gaseous
Acetylene has a pungent odor and this provides certain advantages in case of leakage. Its mass is close to that of atmospheric air.
Liquid
Liquid acetylene has no color. It has one feature: it refracts color. Acetylene, both liquid and gaseous, is a dangerous substance. That is, if the rules for handling it are violated, an explosion can occur at any second, even at room temperature. To increase safety when handling it, so-called phlegmatization is used. That is, a porous substance is placed in a container intended for acetylene storage. Which reduces its danger
Acetylene reactions
Acetylene reacts with various compounds, for example, copper and silver salts. As a result of such interactions, substances called acetylenides are obtained. Their distinguishing feature is their explosiveness.
Acetylene production
Acetylene combustion
Acetylene oxidation reaction
Polymerization reaction
Acetylene substitution reaction
Storage and transportation
All storage and transportation methods involve the use of cylinders. They are filled with a special mass of porous consistency. It is impregnated with acetone, which dissolves acetylene well. The use of this method can significantly increase the filling capacity of an acetylene cylinder and, importantly, reduces its explosion hazard.
Prolonged contact of acetylene with metals such as copper and silver can lead to an increase in its explosion hazard. Therefore, the use of materials that may contain these metals, for example in valves, is prohibited.
As a rule, cylinders must have special valves designed specifically for acetylene storage.
Full use of the entire container capacity can be achieved by storing empty containers so that the acetone is distributed throughout the entire volume of the container. And this is only possible in a horizontal position. Filling the cylinder must occur very slowly, which is important to comply with the conditions of the chemical reaction of dissolving acetylene in acetone, and in particular its speed.
Use of acetylene
In addition to welding, acetylene is used in the following cases:
- to produce bright light in autonomous light sources (carbide lamp);
- in the manufacture of explosives, these are the already mentioned acetylenides;
- obtaining certain chemicals, for example, vinegar, alcohol, polymers, etc.;
- In addition, acetylene has also found its use in rocket technology, as a fuel component.
Cutting metal with acetylene
Use of acetylene in a lamp