Acetylene explosion
Since acetylene combustion and explosion are possible in the absence of oxidizing agents, including oxygen, it, like hydrogen, is the most explosive gas. The similarity with hydrogen is that they have the lowest ignition energy, but more on that later.
The explosiveness of flammable gases and vapors is characterized by an indicator - the amount of ignition energy. A substance with a low value is more explosive. Ignition energy values (MJ) for stoichiometric gas mixtures at atmospheric pressure and temperature 20°C are given in the table below.
| Gas | Mixture with air | Mixture with oxygen |
| Methane | 0,3 | 0,0038 |
| Ethane | 0,25 | 0,0019 |
| Propane | 0,24 | 0,002 |
| Hydrogen | 0,02 | 0,0003 |
| Acetylene | 0,019 | 0,0003 |
From the data in the table it can be seen that the ignition energy of mixtures with air is approximately 100 times greater than that of mixtures with oxygen.
Pure acetylene is capable of exploding upon rapid heating to 450-500°C and excess pressure over 1.5 kgf/cm2. The most explosive mixtures with air are those containing from 7 to 13% C2H2. If C2H2 is present in a mixture with air from 2.2 to 81% by volume, the mixture explodes at atmospheric pressure.
Also, at atmospheric pressure, a mixture of oxygen and acetylene from 2.8 to 93% by volume is explosive. The most explosive mixture with oxygen contains 30% C2H2. Therefore, strong local heating, a flame, and even a spark can cause an explosion of a mixture of acetylene with oxygen or air.
The explosiveness of pure acetylene is determined by pressure, temperature, and also depends on its purity, moisture content, the presence of catalysts, the nature of the explosion agent, the size and shape of the vessel, heat removal conditions and a number of other reasons. As the pressure increases, the molecules of acetylene gas come closer together, which facilitates the spread of decomposition throughout the entire mass of gas. This is confirmed, on the one hand, by the fact that liquid acetylene, in which the proximity of molecules is especially great, is a highly explosive substance even at ordinary temperatures. On the other hand, compressed acetylene loses its explosiveness if its molecules are in any way separated from each other. This is achieved by mixing acetylene with nitrogen or inert gases that do not interact with it, as well as by absorbing acetylene with acetone or other solvent in the presence of a porous substance. For example, wet acetylene is less explosive than dry acetylene. A mixture containing 1.15 volumes of C2H2 per volume of water vapor is not capable of explosive decomposition. This can be explained similarly to what was said above by the separation of C2H2 molecules by water vapor.
The explosion of acetylene or its mixture with oxygen and air is accompanied by the release of heat, resulting in an increase in pressure and temperature. Consequently, such explosions can cause destruction and accidents. Therefore, handling acetylene requires strict adherence to safety measures.
The pressure generated during an explosion depends on the initial parameters and nature of the explosion, and increases by approximately 10-15 times compared to the initial pressure
When acetylene is dissolved in liquids, its explosiveness decreases. It dissolves best in acetone, but more about this in the article on polymerization and dissolution of acetylene
With prolonged contact of C2H2 with copper, silver and mercury, explosive compounds are formed - acetylenoids. Acetylenoids explode on impact or when heated above 100°C. Therefore, for the manufacture of equipment in contact with acetylene, alloys with a copper content of no more than 70% are used.
That is why all equipment for transporting and storing acetylene is made of steel and has a specific design that excludes the possibility of connecting equipment for other gases.
The valve of the acetylene cylinder does not have a connecting thread and is opened with a specially designed socket wrench, and the reducer is connected with a clamp.
When chlorine reacts with acetylene containing even a small amount of air, an explosion occurs. Pure acetylene, when in contact with chlorine, explodes under intense light.
Properties and scope
Mass of 1 m³ of gas at a temperature of 20°C and a pressure of 760 mm Hg. Art. equals 1.09 kg. The decay of 1 kg of a substance releases a large amount of heat (8473.6 kJ). Therefore, acetylene is widely used in technological processes requiring high temperature exposure. In addition, it is the only gas that burns even in the absence of oxygen or any other oxidizing agent.
Acetylene gas can dissolve in many liquids, including acetone, gasoline, benzene and water. To do this, the container is filled with a porous mass, which is soaked in a solvent (usually acetone). In the pores, C2H2 dissolves in acetone, and when the valve is opened, it is released as a gas.
This hydrocarbon is produced in two grades: A and B. The first is used in lighting installations, and the second is used in gas-flame processing of metal products. This is one of the most popular gases used in welding production, along with argon and carbon dioxide. By the way, you can read a lot of useful information about carbon dioxide in the article: Carbon dioxide: where to refuel is not an idle question.
The figure shows the molecular structure of this substance
Acetylene toxicity
The toxicity of acetylene is due to the presence of harmful impurities in it. Acetylene causes drowsiness, and the harmful impurities contained in it, with prolonged inhalation, poison the body and cause nausea, dizziness, and sometimes severe general poisoning.
Therefore, the rooms where acetylene is produced and where work is carried out using it must have good ventilation.
Text of the book “Welding works. Practical guide"
Materials used in gas welding
Combustible gases
In gas welding, oxygen is used as an oxidizing agent, and acetylene, hydrogen, propane, etc. are used as flammable gases.
Oxygen gas
(O2) is colorless, odorless and tasteless, slightly heavier than air. The density of oxygen at atmospheric pressure and temperature 20 °C is 1.33 kg/m3. Actively supports combustion and serves to increase the temperature of the gas flame during combustion of combustible gas.
According to GOST 5583–78, the industry produces gaseous technical oxygen of two grades. The volume fraction of oxygen in technical oxygen of grade I is 99.7%, grade II – 99.5%.
Oxygen is capable of forming explosive mixtures with flammable gases or vapors of liquid flammable substances, and when it comes into contact with organic compounds (oils, fats and other substances), they can spontaneously ignite.
Acetylene gas
(C2H2) is a colorless gas that has a specific garlic odor due to the presence of impurities: hydrogen phosphorous, hydrogen sulfide, etc. Acetylene is lighter than air: at atmospheric pressure and a temperature of 20 °C its density is 1.09 kg/m3. It dissolves well in liquids, especially acetone, becoming safer. Used to form a gas flame during combustion in a stream of oxygen. The advantage of acetylene over other flammable gases is the ability to obtain the highest flame temperature (up to 3200 °C).
At the welding site, acetylene is produced in gas generators by decomposing calcium carbide with water or using pyrolysis [15]15
Acetylene produced from natural gas is called pyrolysis.
[Close] acetylene. The latter is delivered to the welding site dissolved in acetone in the form of a porous mass enclosed in a steel cylinder. Pyrolysis acetylene is cheaper than that produced from calcium carbide.
Acetylene forms explosive mixtures with oxygen in the air at normal atmospheric pressure. The most explosive mixtures are those containing 7–13% acetylene. Acetylene can explode without an oxidizing agent!
Hydrogen
(H2) at atmospheric pressure and temperature 20 °C is a flammable gas, colorless and odorless. The density of hydrogen is 0.084 kg/m3, it is 14.5 times lighter than air. Hydrogen is intended to form a gas flame when burned in a stream of oxygen. The flame temperature is 2600 °C. The hydrogen-oxygen flame is colorless and does not have clear outlines, which makes it difficult to regulate.
It is stored and supplied in a gaseous state in steel cylinders of 5, 10, 20 and 40 liters.
Hydrogen forms an explosive explosive mixture with oxygen (2 volumes of hydrogen and 1 volume of oxygen).
Technical propane
is a mixture of propane (C3H8) and propylene (C3H6), which under normal conditions is a colorless, odorless gas. For safe use, strong-smelling substances - odorants - are added to the mixture. The gas is heavier than air; at atmospheric pressure and a temperature of 20 °C its density is 1.88 kg/m3. Used to form a gas flame with a temperature of 2700 °C as a substitute for acetylene.
Propane is supplied to the welding site in all-welded steel cylinders in a liquefied state.
Propane is flammable. Can accumulate in pits, basements and wells, forming an explosive mixture.
MAF gas
– methylacetylene-allen gas fraction, formed during the processing of natural gas and petroleum products, which has good thermophysical properties. The gas is heavier than air, its density under normal conditions is 1.9 kg/m3. It has a pronounced odor.
MAF gas is used as a substitute for acetylene in gas welding. It is half the price of acetylene, and the flame temperature during its combustion reaches 2930 °C. Gas is supplied to the welding site in a liquefied state in all-welded cylinders (the same as for propane). A cylinder with a capacity of 50 liters and a weight of 22 kg contains 21 kg of gas.
The tendency for MAF gas to backfire is negligible. Compared to acetylene, MAF has a softer flame, which gives its advantages when working with thin metals, non-ferrous metals, and also when contour cutting products. At the same time, the core of even a neutral flame when using MAF gas is 1.5–2 times longer than acetylene.
The technology of gas flame processing when using MAF gas is basically the same as when using acetylene. The equipment can be used burners, cutters, gearboxes and other devices designed to work with acetylene and liquefied gases (propane-butane mixtures). It is better to use filler wire that is more suitable for propane welding.
A gas cylinder can use a reducer used on propane cylinders. Compared to a propane-butane mixture, when welding steel with MAF gas, the oxygen consumption is 1.5 times less.
A mixture of MAF gas (3.4–10.8% by volume) with air is explosive.
Gas can accumulate in basements, wells and pits, forming an explosive mixture. Filler materials
Filler materials are wire, rods (rods), strips of metal that are similar in properties to the metal being welded. When welding, they provide additional metal to fill the gap between the welded edges and form the weld of the required shape.
The main filler material is welding wire.
When welding carbon and alloy steels
Cold drawn welding wire is used. Its characteristics are given above, in the chapter “Characteristics, classification and purpose of welding wire”.
For gas welding of gray cast iron
They produce cast iron rods ∅ 4, 6, 8, 10, 12 and 16 mm. The end of the rods is marked with black, white, red, blue, brown, yellow or green paint.
For gas welding of copper, copper-nickel alloys, bronze and brass
use welding wire that meets GOST 16130–90. Its diameter is 0.8–8.0 millimeters.
The symbol for filler wire made of copper or its alloy corresponds to the classification of these materials according to the following criteria:
● manufacturing method (cold-deformed (drawn) – D
;
hot-deformed (pressed) – Г
);
● section shape – KR
(the wire is made exclusively of round cross-section);
● mechanical properties (soft – M
, hard –
T
);
● type of delivery (coils or coils - BT
, coils -
KT
, drums -
BR
, cores -
CP
, unmeasured length -
ND
).
When welding aluminum and its alloys
They use drawn and pressed wire made of aluminum and aluminum alloys that meet GOST 7871–75. Its diameter is 0.8–12.5 mm. Labeling symbols characterize:
● manufacturing method (drawn – B
, pressed –
P
);
● type of processing (hard-worked – N
, annealed –
M
);
● type of delivery (coils (coils) – BT
, coils –
CT
).
Welding flux
[16]
16
The main characteristics of welding fluxes are given above in the corresponding chapter. The characteristics of fluxes used in gas welding of various metals are given below in the section “Features of welding of various metals”.
[Close]. In gas welding, flux is applied to the edges being welded or introduced into the weld pool with the melted end of the filler rod (which sticks to it when immersed in the flux). Fluxes can also be used in gaseous form when supplied to the welding zone with flammable gas.
Gas welding equipment
Equipment for gas welding, surfacing and cutting includes gas supply sources (acetylene generators or gas cylinders), control and protection equipment (valves, gearboxes, pressure gauges, safety devices), connecting hoses and universal or specialized torches.
Acetylene generators
An acetylene generator is a device designed to produce acetylene gas by decomposing calcium carbide with water.
From 1 kg of calcium carbide, depending on the size of its pieces and the degree of purity, 235–285 dm3 of acetylene can be obtained. However, the use of gas generators in everyday life and in small workshops is inappropriate: they are more explosive than cylinders, and therefore the number of safety devices in the welding kit increases. Their maintenance is more difficult; preparation for work takes much longer than when working with cylinders; waste sludge is drained only into special pits or concrete storage facilities. In addition, upon completion of even minor welding work, the entire loaded volume of calcium carbide should be exhausted - gradually vent the acetylene into the atmosphere or burn it with a torch. Therefore, the use of acetylene generators is justified only for industrial volumes of work. Cylinders
A cylinder is a metal container for storing and transporting gases in compressed, dissolved and liquefied states.
Oxygen balloon
, according to GOST 949–73, is made of carbon (150U) or alloy (150JI) steel and has a seamless steel cylindrical body with a convex bottom onto which a shoe is pressed (Fig. 21,
a
). At the top, the cylinder ends with a neck with a threaded hole into which a shut-off valve is screwed. A safety cap is screwed onto the external thread of the cylinder neck.
Rice. 21.
Gas cylinders for welding:
A
– oxygen cylinder with a capacity of 40 l (
1
– bottom;
2
– shoe;
3
– body;
4
– neck;
5
– valve;
6
– safety cap);
b
– oxygen cylinder with a capacity of 10 dm3;
c
– acetylene cylinder (
1
– body;
2
– valve;
3
– nitrogen cushion;
4
– porous mass with acetone;
5
– shoe;
6
– safety cap);
g
– propane cylinder with a capacity of 55 dm3 (
1
– nameplate;
2
– body;
3
– bottom;
4
– shoe;
5
– backing rings;
6
– neck;
7
– valve;
8
– safety cap)
The height of a standard 40–150U cylinder is 1370 mm, diameter 219 mm, wall thickness 7 mm, capacity 40 dm3, weight without gas 67 kg. The cylinder is designed for a working pressure of 15.0 MPa (150 kgf/cm2); the test pressure is 22.5 MPa (225 kgf/cm2). In a full cylinder, the volume of oxygen corresponding to atmospheric pressure and a temperature of 20 °C is 6 m3.
The color of the cylinder is blue, the inscription is black.
Along with cylinders with a capacity of 40 dm3, cylinders with a smaller capacity are also produced - 20; 10; 5 and 1 dm3 (Fig. 21, b).
The oxygen cylinder valve is made of brass, since steel actively corrodes in a compressed oxygen environment, and the flywheels and plugs are made of steel, aluminum alloys and plastic.
The amount of oxygen in the cylinder is approximately determined by solving the following proportion: at atmospheric pressure (0.1 MPa) there is 40 dm3 of gas in the cylinder; if the pressure in the cylinder is 15 MPa, then (40 × 15)/0.1 = 6000 dm3, or 6 m3, of oxygen can be compressed to a volume of 40 dm3.
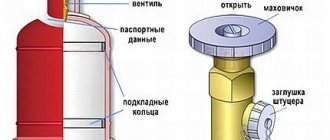
Acetylene cylinder
large capacity has the same dimensions as an oxygen tank with a capacity of 40 dm3 (Fig. 21,
c
). The mass of the cylinder without gas is 83 kg, the working pressure of acetylene is 1.9 MPa (19 kgf/cm2), the maximum pressure is 3.0 MPa (30 kgf/cm2).
An acetylene cylinder is filled with a porous mass of activated charcoal, which is impregnated with acetone at the rate of 225–300 g per 1 dm3 of cylinder capacity. Acetylene, soluble in acetone, becomes less explosive.
More economical are cylinders with cast porous mass, capable of holding 7.4 kg of dissolved acetylene, while cylinders with activated carbon - only 5 kg.
On the cylinder with a cast porous mass, below the inscription “ACETYLENE”, the letters LM are painted in red paint. Such cylinders are supplied with a nitrogen cushion.
When acetylene is removed from the cylinder, part of the acetone in the form of vapor is also removed. To reduce acetone losses during operation, it is necessary to place the cylinders in a vertical position and withdraw acetylene at a rate not exceeding 1.7 m3/h.
In a filled cylinder with a capacity of 40 dm3 at operating pressure and air temperature of 20 °C, the volume of acetylene gas corresponding to normal conditions is 5.5 m3.
The color of the cylinder is white, the inscription is red. Acetylene is also dispensed in cylinders with a capacity of 1; 5; 10; 15 and 20 l.
A distinctive feature of the acetylene cylinder valve is the absence of a flywheel and fitting. The valve body has a side groove into which the acetylene reducer fitting is installed, pressing it with a special clamp through a leather gasket. This valve design does not allow the accidental installation of another gearbox to avoid the formation of an explosive mixture.
Another distinctive feature of the acetylene cylinder valve is that its opening, closing and connection of the gearbox to the cylinder is carried out with a special socket wrench.
To determine the volume of acetylene, the cylinder is weighed before and after filling with gas, and the volume of gas in the cylinder is determined from the difference in acetylene indicators and density. For example, the mass of a cylinder with acetylene is 89 kg, empty - 83 kg. Mass of acetylene in the cylinder: 89–83 = 6 kg. The density of acetylene at atmospheric pressure and a temperature of 20 °C is 1.09 kg/m3. Therefore, the volume of acetylene under these conditions is 6/1.09 = 5.5 m3.
Cylinders for technical propane
made from sheet carbon steel 3 mm thick in accordance with GOST 15860–84.
The neck is welded to the upper part of the welded cylindrical body of the propane cylinder, and the bottom and shoe are welded to the lower part (Fig. 21, d
). The neck has a threaded hole into which a brass valve is screwed. There are cushion rings inside the cylinder. A safety cap is used to protect the cylinder valve from mechanical damage.
Cylinder height no more than 1013 mm, diameter (without reinforcement shell) 292 mm, weight without gas 22 kg, capacity 50 l, operating pressure 1.6 MPa (16 kgf/cm2). The gas in the cylinder is in a liquefied state.
In addition, they produce propane cylinders with a capacity of 27, 12 and 5 liters. A 50-liter cylinder contains 21.4 kg of liquefied gas, a 27-liter cylinder contains 11.4 kg, and a 5-liter cylinder contains 3.3 kg.
The short-term maximum gas withdrawal should not exceed 1.25 m3/h, and the normal one, in order to avoid freezing of the valve, should be 0.6 m3/h.
The color of the cylinder is red, the inscription is white.
The valve of a membrane-type propane cylinder is made of brass (less commonly, steel).
It is designed for operating pressure up to 2.0 MPa (20 kgf/cm2). The side fitting of the valve body has a left-hand thread. Gas reducers and pressure gauges
Reducer
– a device designed to reduce the gas pressure coming from a cylinder and automatically maintain a given operating pressure. Gas reducers also regulate the operating pressure and protect the cylinder from backfire, and pressure gauges show the gas pressure in the cylinder and at the outlet of the reducer.
Gas reducers, according to GOST 13861–89, are classified by purpose (B - cylinder, R - ramp, C - network); type of gas being reduced (A – acetylene, K – oxygen, M – methane, P – propane-butane); control scheme (O, D – one- and two-stage with mechanical pressure setting, Z – single-stage with pneumatic setting of working pressure). They also differ in the principle of action (direct and reverse action). Reverse-action gearboxes are more convenient to operate, as they are compact and simple in design, reliable and safe to operate.
The gearboxes differ from each other in the color of the housing (acetylene - white, oxygen - blue, propane - red) and the connecting devices for attaching them to the cylinder. Oxygen and propane reducers are connected to the cylinders with union nuts with right-hand and left-hand threads, respectively. Acetylene reducers are attached to the cylinders with a clamp with a thrust screw.
Technical characteristics of balloon reducers are given in table. 8 (see p. 326).
Pressure gauges
are instruments for measuring gas pressure. They are attached to the gearbox housing through fiber and leather gaskets using threaded connections using a wrench.
Each pressure gauge must have on the dial a designation of the gas for which it is intended.
The inscriptions “Oxygen” and “Oil Dangerous” are applied to oxygen pressure gauges; on acetylene, hydrogen and propane - “Acetylene”, “Hydrogen” and “Propane” or the symbols O2, C2H2, H2 and C3H8. Hoses
are flexible pipelines used to transport gas to the work site and supply it to the burner. Depending on the purpose, rubber hoses for gas welding are divided into three classes:
● I – for supplying acetylene, city gas, technical propane and other flammable gases under pressure up to 630 kPa (6.3 kgf/cm2); sleeves are red;
● II – for supplying liquid fuel (gasoline, white spirit, kerosene or mixtures thereof) under pressure up to 630 kPa (6.3 kgf/cm2); sleeves are yellow;
● III – for supplying gaseous oxygen under pressure up to 2.0 MPa (20 kgf/cm2); The sleeves are blue.
The sleeves are made of rubber reinforced with layers of fabric. Oxygen hoses have inner and outer layers of vulcanized rubber and several layers of linen or cotton fabric.
The hoses are used at ambient temperatures from –35 to +50 °C. To work in northern latitudes, hoses made of frost-resistant rubber are required, which retain their properties at temperatures down to –65 °C.
Hoses of classes I and II have a four-fold, and class III – a three-fold safety factor in relation to the working pressure.
The sleeves are made with an internal diameter of 6.3;
8.0; 9.0; 10.0; 12.0; 12.5 and 16.0 mm. Hose lengths of 10 and 20 m are supplied in the form of coils. The optimal hose length is 9–30 m. As it increases, gas pressure losses increase. Welding torches
The main tool of a gas welder is a welding torch - a device for mixing gases, forming a welding flame and regulating its type and power. Welding torches are classified according to the following criteria:
● method of supplying flammable gas and oxygen to the mixing chamber - injection and non-injector;
● type of flammable gas – acetylene, hydrogen, for substitute gases and liquid combustibles;
● number of torches – single-flame and multi-flame;
● purpose – universal (welding, cutting, soldering, surfacing) and specialized for performing one operation;
● flame power – micro-, low-, medium- and high-power burners;
● method of application – manual, machine.
In non-injector burners, combustible gas and oxygen enter the mixer at the same pressure. Injector burners have a device that supplies low-pressure combustible gas to the mixing chamber by sucking it into a stream of oxygen supplied under higher pressure. This device is called an injector, and the suction phenomenon is called injection. The most effective are injection burners, characterized by high safety, ease of maintenance, reliable operation and versatility.
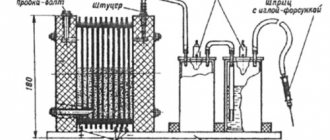
In Fig. 22, a – c
The diagram of the injection burner and the design of the injection device are presented. Oxygen from the cylinder under operating pressure through the nipple, gas supply tube and valve enters the injector nozzle. Coming out of the nozzle at high speed, it creates a vacuum in the acetylene channel, as a result of which acetylene, passing through the nipple, tube and valve, is sucked into the mixing chamber. A flammable mixture is formed in this chamber, which, passing through the tip and mouthpiece, burns at the outlet of the torch, forming a welding flame.
For normal operation of injection burners, it is necessary that the oxygen pressure be 150–500 kPa (1.5–5.0 kgf/cm2), and the acetylene pressure be 3–120 kPa (0.03–1.2 kgf/cm2). Stable flame combustion is achieved at a flow rate of the combustible mixture of 50–170 m/s.
In Fig. 22, d
A diagram of a non-injector burner is presented.
Instead of an injector, it has a mixing chamber at the tip. When connecting a non-injector burner to gas cylinders, a reducer is used that automatically maintains equality of the operating pressures of oxygen and acetylene (Fig. 22, d
).
Rice. 22.
Burners:
a – c
– injection (general view, design of the burner and injector;
1
– mouthpiece;
2
– tip;
3
– mixing chamber;
4
– injector nozzle;
5, 7
– oxygen and acetylene valves;
6
– nipples;
8, 9
– channels for oxygen supply and acetylene;
10
– injector);
d
– diagram of connecting a non-injector burner to gas cylinders;
e
– design of a non-injector burner (
1
– mouthpiece;
2
– tip;
3, 6
– oxygen and acetylene valves;
4, 5
– oxygen and acetylene nipples;
7, 8
– balloon reducers;
9
– equal pressure reducer;
10
– hoses;
11
– burner,
12
– safety devices);
e
– injection cutter (
1
– oxygen cutting jet valve;
2
– oxygen supply tube to the mouthpiece;
3
– heating flame;
4
– oxygen cutting jet)
Oxygen flows through the nipple, control valve and special metering channels into the mixing chamber of the burner. Similarly, acetylene is supplied through the nipple and valve. From the mixing chamber, the combustible mixture passes through the tip and exits the mouthpiece, forming a welding flame.
The flame power is selected depending on the thickness of the metal being welded and its thermophysical properties. Adjust the flame by selecting the burner tip. The rules for choosing a welding torch and tips for it are given in table. 9 and 10.
Autoignition of acetylene
The auto-ignition temperature of acetylene ranges from 240-630°C depending on the pressure and the presence of various substances in it. It decreases with increasing pressure or with the presence of other substances in acetylene that increase the contact surface.
The dependence of the auto-ignition temperature on pressure is presented in the table below.
| Temperature, °C | Pressure, MPa |
| 630 | 0,2 |
| 530 | 0,3 |
| 475 | 0,4 |
| 350 | 2,2 |
In practice, depending on the pressure, it is permissible to heat acetylene to temperatures:
- up to 300°C - at an absolute pressure of 0.1 MPa (1 kgf/cm2);
- up to 150-180°C - at an absolute pressure of 0.25 MPa (2.5 kgf/cm2);
- up to 100°C - at higher pressures.
When burning acetylene mixed with oxygen in injection burners and cutters, it is necessary to take into account the possibility of flame penetration into the acetylene channel and its propagation in the direction opposite to the normal gas flow. This phenomenon is called flashback and can cause an explosion in the acetylene line or generator.
ACCEPTANCE RULES
2.1. Dissolved and gaseous acetylene is taken in batches. A batch of dissolved acetylene is considered to be any quantity of acetylene that is homogeneous in its quality indicators, obtained in one technological cycle and accompanied by one quality document.
A batch of acetylene gas transported through a pipeline is any amount of acetylene sent to the consumer within 24 hours.
A batch of dissolved and gaseous acetylene sent to the consumer must be accompanied by a quality document containing the following data:
name of the manufacturer and its trademark;
name, brand and grade of product;
batch number;
date of manufacture of the product;
amount of acetylene in kilograms or cubic meters;
results of analyzes performed or confirmation of product compliance with the requirements of this standard;
designation of this standard.
(Changed edition, Amendment No. 2).
2.2. A sample of dissolved acetylene at manufacturing plants is taken from each batch from the collector after drying the acetylene before the filling ramp. The sample volume is 20 - 35 dm3.
2.3. A sample of acetylene gas is taken at least once every 24 hours directly from the pipeline in front of the central water seal.
The sample volume should not be less than 20 dm3.
2.4. To check the quality of dissolved acetylene, the consumer selects 2% of the cylinders, but not less than two cylinders for a batch volume of less than 100 cylinders. The pressure in selected cylinders is checked.
2.5. If unsatisfactory analysis results are obtained for at least one of the indicators, a re-check should be carried out on a double sample or a double volume of samples from the same batch.
The results of repeated tests are final and apply to the entire batch.
Safety precautions when working with acetylene
Now, knowing how acetylene is dangerous, it became clear why special safety measures must be observed when working with it.
The basic safety requirements when working with this gas are set out in GOST 12.2.054, RD 34.03.204 and other regulatory documents, but here we will only briefly consider the most important points:
- It is prohibited to store oxygen cylinders in the same room as acetylene cylinders.
- On a special hand trolley, it is allowed to transport no more than one oxygen cylinder and one acetylene cylinder directly to the workplace.
- It is prohibited to completely use all the gas from the cylinder. The acetylene cylinder must have a residual pressure of at least:
Temperature, °C below 0 0-15 16-25 26-35 Minimum permissible residual pressure according to the pressure gauge, MPa (kgf/cm2) 0.049 0,049 (0,5) 0,098 (1,0) 0,196 (2,0) 0,294 (3,0) - In order to reduce losses of acetone, in which acetylene is dissolved, gas is taken from cylinders located only in a vertical position.
- Acetylene cylinders are allowed to be stored in special warehouses or in open areas under a canopy that protects them from precipitation and direct sunlight.
- For the manufacture of parts that come into direct contact with acetylene, the use of:
- copper and alloys in which the copper content is more than 65%;
- silver and its alloys;
- mercury;
- magnesium;
- zinc;
- and etc.
Dissolved acetylene during gas-flame work
This hydrocarbon is considered a universal combustible and is widely used in flame processing, cutting and welding of metals. Since autogenous and welding works are an integral part of most technological processes in the construction and industrial sectors, its value is difficult to overestimate.
The main task of acetylene is the high-temperature effect on metal. The oxy-acetylene flame can exceed 3000°C, resulting in high productivity and welding quality. This is its main difference from another common welding gas - propane, whose flame temperature is slightly above 2000°C. If you want to learn more about propane, then read the article: why propane and butane are mixed - properties of liquefied petroleum gases.
This is what the flame looks like when welding using this gas.
The welding station is supplied with the help of acetylene generators or cylinders, which, as noted above, are filled with a porous mass. This is done in order to divide the total volume of solution into many cells, and thereby significantly reduce the likelihood of flame propagation and explosion. In addition, the porous structure increases the contact area between gas and acetone, which makes it possible to increase the amount of hydrocarbon in the container.
By the way, you will find information about other technical gases and their applications in the corresponding section of the blog.
Storage and transportation
The dissolved composition is stored in white steel cylinders (usually 40 l) with a characteristic red inscription, which are equipped with special acetylene valves. The internal pressure at +20°C should not be higher than 1.9 MPa, so 40-liter cylinders are filled with no more than 5.8 kg of acetylene.
Transportation can be carried out by all types of vehicles, in accordance with the rules for the transportation of dangerous goods that apply to this type of transport, as well as the rules for the safe operation of pressure vessels.
If you need to refill acetylene cylinders, contact us, where you will receive professional service and affordable prices for gas products.