C2H2 acetylene. Properties of acetylene. Preparation of acetylene.
Properties of acetylene.
The most widely used flammable gas for oxy-fuel cutting is acetylene, which is a chemical compound of carbon and hydrogen ( C2H2 ).
At normal temperature and pressure, acetylene is in a gaseous state. Chemically pure acetylene is a colorless gas with a weak ethereal odor and a slightly sweet taste. Technical acetylene, used for oxygen cutting, due to the presence of certain impurities in it (hydrogen sulfide, ammonia, hydrogen phosphorous, etc.), has a strong unpleasant odor and is harmful to the human body.
At a pressure above 2 kg/cm2, acetylene in large volumes acquires the properties of an explosive gas and, upon contact with a heated surface or from an electric spark, explodes, with the temperature reaching 3000°C and the pressure increasing by more than 10 times.
With oxygen and air, acetylene forms explosive mixtures that explode when exposed to fire. Acetylene - air mixture is explosive in the presence of from 2.3 to 81% acetylene in the air. Even more dangerous is the acetylene-oxygen mixture, which explodes from fire when the oxygen content is from 2.3 to 93% acetylene, while the speed of propagation of the blast wave reaches 3000 m/sec, so you need to especially carefully monitor the density of all acetylene devices and pipelines etc.
Also read: What are the properties of oxygen. Weight of 1 m3 of oxygen. Weight of liquid oxygen. Methods for obtaining oxygen.
The explosiveness of acetylene is greatly reduced when placed in capillary (very thin) channels. This property of acetylene is used when filling cylinders under pressure - acetylene cylinders are filled with a special porous mass.
Reaction of acetylene with acetone.
Acetylene is highly soluble in many liquids, especially acetone . In one volume of acetone at 15°C and normal pressure, 23 volumes of acetylene are dissolved, and at elevated pressure - proportionally more. This property of acetylene is also used when filling cylinders. The solubility of acetylene in acetone largely depends on temperature: with increasing temperature, the solubility decreases, and with decreasing temperature it increases sharply.
The ability of dissolved acetylene to explode is reduced.
Acetylene combustion reaction.
Acetylene, when burned in a mixture with pure oxygen, produces a flame with a temperature of 3050 - 3150 ° C, and when burned in a mixture with air, it produces a flame with a temperature of 2350 ° C.
Complete combustion of acetylene.
For complete combustion of 1 m3 of acetylene, 2.5 m3 of oxygen or 12.5 m3 of air is required.
Acetylene is lighter than oxygen and air. Specific gravity of acetylene at 0°C and 760 mm Hg. Art. 1.17 kg/m3.
With prolonged interaction with red copper and silver, acetylene produces compounds that explode when heated or upon strong impact. Therefore, in the manufacture of acetylene equipment and fittings, the use of silver solders and red copper in their pure form is prohibited. Only technical copper alloys containing no more than 70% copper are allowed.
Preparation of acetylene.
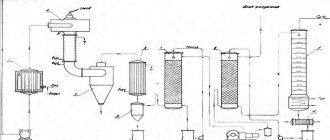
The main industrial method for producing acetylene is the decomposition of calcium carbide with water. Calcium carbide is a chemical compound of calcium with carbon ( CaC2 ) and is a dark gray or grey-colored solid.
Technical calcium carbide is obtained by fusing quicklime with coal (coke or anthracite). The charge, consisting of a mixture of coal and lime in a certain proportion, is immersed in an electric arc furnace, where, under the influence of the heat of the electric arc, it melts to form calcium carbide.
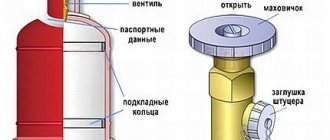
Molten carbide is poured from the furnace into molds, in which it solidifies. The solidified calcium carbide is then crushed, sorted into pieces of certain sizes and packaged in special drums for storage and transportation. Drums for packaging calcium carbide are made of sheet iron with a thickness of at least 0.5-0.6 mm with a hermetically sealed lid. Corrugations are rolled onto the cylindrical surface of the drums to increase their strength. The drum holds 50-130 kg of calcium carbide.
Carbide drums should be opened very carefully, since for one reason or another moisture may penetrate inside the drum and an explosive acetylene-air mixture may form. It is best to cover the drum cover with a layer of grease and remove it with a special knife . Under no circumstances should you resort to hammer blows.
When uncorking carbide drums, it is strictly forbidden to use a flame or cut out the drum cover with a chisel.
Acetylene decomposition.
When exposed to water, calcium carbide decomposes, forming acetylene and slaked lime. The reaction proceeds according to the equation
CaC2 + 2H2O = C2H2 + Ca(OH)2
and is accompanied by significant heat release.
Calcium carbide, when in contact with atmospheric air containing water vapor, decomposes with the release of acetylene. From 1 kg of calcium carbide usually produces from 230 to 300 liters of acetylene.
The decomposition of 1 kg of calcium carbide theoretically requires 0.56 liters of water . The decomposition reaction of carbide proceeds with the release of a large amount of heat, so a lack of water can lead to significant overheating and even ignition of acetylene at the site of carbide decomposition. To avoid this, the carbide decomposition process is always carried out with an excess amount of water, which is used for cooling. Almost 1 kg of calcium carbide requires 5 to 20 liters of water.
Was the article useful?! Share with your friends on social networks!!!
Acetylene
Details Category:
ACETYLENE
, C2H2. Molecular weight 26. Acetylene in its physical properties is a colorless gas, in its pure state it has a weak, peculiar odor; somewhat lighter than air (specific gravity 0.9056). 1 liter of acetylene weighs about 1.17 g. Soluble in water, in alcohol and very easily, under pressure, in acetone; 1 volume of acetone dissolves about 300 volumes of acetylene under a pressure of 12 atm; the liquid greatly increases in volume. Acetylene at 25° and 94 atm condenses into a colorless, easily mobile liquid, boiling under atmospheric pressure at -83°.8, specific gravity 0.451; critical temperature +37°, critical pressure 68 atm. With further cooling of liquid acetylene, it solidifies into a white snow-like mass. Since the melting point is very close to the boiling point, such solid acetylene evaporates without melting - it sublimes.
Chemically, acetylene is an unsaturated hydrocarbon, which is the first member of a homologous series of similar hydrocarbons. All of them are characterized by the presence in the particle of the so-called triple bond between carbon atoms and have the general formula CnH2n-2 (n = 2, 3, 4, ...).
The structure of acetylene is represented by the formula CH:CH
. According to the concepts developed in organic chemistry, compounds of this kind are less strong than saturated compounds that do not contain multiple bonds (double or, as in acetylene, triple). These compounds are endothermic. The formation of acetylene can be represented by the following equation: 2C + 2H = C2H2 - 61 cal. Acetylene, like all endothermic compounds, can decompose explosively under suitable conditions. However, at ordinary pressure it is safe and acquires the properties of an explosive only when the pressure increases above 2 atm or in liquid form. Mixtures of acetylene with inert gases or its solutions are much safer. Acetylene burns with a glowing and smoky flame in air. With complete combustion, 1 volume of acetylene produces 2 volumes of carbon dioxide and 1 volume of water vapor. For complete combustion of 1 volume of acetylene, theoretically, 2.5 volumes of oxygen or 12.5 volumes of air are required. The molecular heat of combustion of acetylene is 312.9 cal. at constant pressure. 1 liter of acetylene at 0° and 760 mm has a calorific value of 14100 cal. The temperature of the luminous flame is about 1900°; the combustion temperature of a mixture of 0.6 volumes of acetylene and 1 volume of oxygen (usual ratio for autogenous welding) is about 2400°; The flash point of mixtures of acetylene with air and oxygen lies in the range of 400-500°. At temperatures of 650-800°, acetylene partly decomposes into elements and partly polymerizes to form liquid and solid hydrocarbons. At temperatures above 1500°, ethylene and ethane are formed.
Mixtures of acetylene with oxygen and air are extremely explosive. Mixtures with air containing more than 5 and less than 80% acetylene explode from a flame, electric spark, etc. As an unsaturated compound, acetylene is characterized by its ability to undergo addition reactions. Halides (chlorine, bromine) react vigorously with acetylene, which means the reaction must be carried out in solutions, with the formation of halogenated hydrocarbons: ethane and ethylene. Hydrogen iodide and hydrogen bromide also add directly to acetylene. The addition of hypochlorous acid (HClO) even in aqueous solutions can be accompanied by an explosion.
Dilute aqueous solutions of hypochlorites (salts of hypochlorous acid, for example, bleaching lime) have almost no effect on acetylene, concentrated ones react violently. Dry bleaching lime in the cold acts only on impurities of technical acetylene, which is what is used to clean it, but always with the addition of excess alkalis (to avoid the possibility of the formation of explosive compounds). Acetylene can, in the presence of catalysts (Ni), add hydrogen, forming ethylene, ethane and a number of liquid hydrocarbons. Nitric acid oxidizes acetylene; in this case, part of the process involves the addition of water and nitration; some of the resulting products are explosive. Alkaline solutions of potassium permanganate oxidize acetylene to carbonic anhydride, partly to formic and oxalic acid. When chromic acid reacts with acetylene, acetaldehyde and acetic acid are formed. Aqueous solutions of chromic acid have no effect on acetylene, which can be used to purify technical acetylene. By passing acetylene in a mixture with ammonia over aluminum oxide, pyrroles and pyridine bases (Chichibabin) are obtained at a temperature of 300-400°. When acetylene is passed over pyrite (sulfur pyrite), thiophene and its homologues (Steinkopf) are formed. Acetylene adds elements of water to form acetaldehyde when passing it into sulfuric acid containing mercuric oxide salts (Kucherov). Characteristic of acetylene is its ability to form water-insoluble explosive precipitates of derivatives of copper, silver and some other metals. A red precipitate of acetylene copper (composition CCu2, sometimes CCu2∙H2O) is obtained by passing acetylene through solutions of (neutral) copper salts or through an ammonia solution of copper oxide. When dry, the sediment explodes for insignificant reasons. Silver and mercury acetylides are formed similarly. Wet acetylene in the presence of ammonia (technical acetylene) also acts on metallic copper. Acetylene has no effect on iron, nickel, cobalt, lead, cadmium, platinum, zinc and tin salts. Metal derivatives of copper, silver and mercury are decomposed by acids, releasing back acetylene. A derivative of acetylene is the so-called. calcium carbide CaC2; this calcium acetylide easily decomposes with water to form acetylene and calcium oxide hydrate. Carbide is obtained by fusing lime and coal in an electric furnace (~ 3000°). This reaction is now being reproduced technically on a large scale, and this carbide serves as a source of technical acetylene.
Acetylene was first obtained synthetically by Berthelot; he placed a voltaic arc formed between carbon electrodes in a hydrogen atmosphere. Acetylene is often formed during pyrogenic processes from more complex compounds. Technically, acetylene is now obtained, as stated above, by the decomposition of calcium carbide with water.
Since the introduction of carbide as a mass product in 1895, acetylene has been predicted to have a bright future. It was believed that it would be able to displace lighting gas from everyday use. It didn't come true. The reasons lay in the unpleasant properties of acetylene: its explosiveness, the ability to form explosive compounds with copper as a reinforcement material, the disgusting smell and toxicity of technical acetylene impurities. In addition, the design of the burners required a special design. Acetylene burns with a brilliant and non-smoking flame only if it enters the air under a certain pressure and if the design of nozzles at the burners allows for sufficient mixing with air, and also if decomposition products (graphite) or polymerization of acetylene are not formed in such a nozzle under the influence of high temperature. This problem has been resolved, but acetylene lighting is used only for small or portable installations. The high temperature and small volume of the oxygen-acetylene flame have led to the widespread use of acetylene for the so-called. autogenous welding. Before the World War in Germany, at least 30-40% of carbide went to this branch of acetylene use.
In the chemical industry itself, certain halogen derivatives obtained from acetylene and chlorine were widely promoted and became widespread. These are liquids that perfectly dissolve fats, resins, etc. substances, non-flammable, with low heat of vaporization; they successfully replace flammable gasolines and ether for the extraction of the mentioned substances. The easiest way is to initially obtain tetrachlorethylene, which, however, has some practically inconvenient properties (reacts with alkalis and acts on iron in the presence of water); Therefore, by heating with milk of lime, this product is converted into trichlorethylene C2HCl3 - a liquid with a boiling point of 85°, indifferent even in the presence of water with respect to iron, copper, lead, zinc and tin. The pre-war overproduction of chlorine found in these products a new way to sell excess chlorine on the market. The rise in price of dry wood distillation products, in particular acetic acid necessary for the synthesis of indigo (via phenylglycine from aniline and chloroacetic acid), prompted the use of trichlorethylene to replace acetic acid in this branch of dye synthesis. By heating trichlorethylene with sodium alkoxide, dichlorovinyl ether was obtained according to the scheme: C2HCl3 + NaOC2H5 = NaCl + C2HCl2OC2H5. By heating this latter with water, chloroacetic ether was obtained: C2HCl2OC2H5 + H2O = HCl + ClCH2COOC2H5.
In the post-war years, the following cycle of reactions begins to become increasingly important. Passing acetylene into hot 6% sulfuric acid containing mercuric oxide salts (Kucherov reaction) gives acetaldehyde according to the reaction: C2H2 + H2O = CH3∙CHO. Aldehyde can be oxidized with atmospheric oxygen by dissolving it in ready-made acetic acid; oxidation occurs in the presence of small amounts of manganese salts. The result is synthetic acetic acid CH3∙CHO + O = CH3COOH. It is possible to condense two particles of aldehyde into one particle of acetic ether (Tishchenko reaction) in the presence of aluminum alkoxide: 2 CH3∙CHO = CH3COOC2H5. As you can see, all these reactions, in essence, only need cheap acetylene, because the remaining starting materials - water and atmospheric oxygen - cost nothing, and the catalysts are regenerated during operation. The question of whether acetic acid obtained by this method can compete with acetic acid obtained by dry distillation of wood is a question of the cheapness of acetylene or, ultimately, a question of cheap electrical energy for the production of carbide. In any case, obtaining ethyl acetate in this way is already practiced on a technical scale and is quite cost-effective.
Source: Martens. Technical encyclopedia. Volume 1 - 1927
- < Back
- Forward >