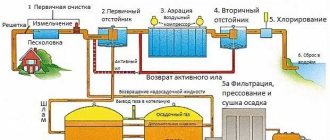
Various anti-corrosion protection technologies are used for metal sheets and parts. Cathodic protection against corrosion has become widespread. This method has a number of characteristic features, and most often cathodic protection is used for large objects. These could be pipes, cars, metal pile structures, sea vessels. How exactly are pipelines protected from corrosion at the physical and chemical level?
Basic cathodic protection technologies
Cathodic protection is a special method of electrochemical protection of metal objects from rust and corrosion. The main principle is that a negative electrical potential is applied to the metal object being protected. This minimizes metal contact with external ions and substances with an electrical charge. The technology was developed approximately 200 years ago by British scientist Humphry Davy. To confirm his theory, he compiled several reports that were submitted to the government. Based on these reports, the world's first cathodic protection of a large industrial ship was carried out.
Anti-corrosion protection applies to various objects - pipelines, cars, roads, airplanes and so on. Please note that the type of metal does not matter - it can be iron, copper, silver, gold, aluminum, titanium and any other metal, as well as various alloys (with or without alloying additives). Corrosion protection of a car, individual pipe fragments, various decorative items of complex shape, and so on can be equally successful.
1 way
Connecting the part to an external source of electric current (usually compact substations perform this role). When the technology is used, the metal object acts as a cathode, and the electrical substation acts as an anode. Due to this, a shift in the electrical potential occurs, which makes it possible to protect the metal object from electrically active particles. The main areas of application of this technology are the protection of pipelines, welded structures, various platforms, road surface elements, and so on. This technology is quite simple and universal, so it is very popular in the world. Its main disadvantage is the need to connect the protective circuit to an external current source, which can be inconvenient in the case of objects that are located far from human civilization (this problem is partly solved through the use of autonomous energy sources).
Method 2
Galvanic polarization method (galvanic anode technology). This technique is also quite simple and intuitive: a metal object is attached to another that has a negative charge (most often this element is made of light metals - aluminum, zinc, magnesium). Galvanic polarization technology is usually used in cases where there is a protective layer on the surface of the object. This technology is popular in America, where there are a large number of sparsely populated areas and where there is a shortage of external energy sources. Experts argue that galvanic polarization could become very popular in Russia due to the peculiarities of our geography if a protective coating were applied to domestic pipelines (in such a scenario, the use of the first technology would be very difficult, forcing people to look for an alternative).
Electrochemical protection of pipelines against corrosion
Electrochemical corrosion protection consists of cathodic and drainage protection. Cathodic protection of pipelines is carried out by two main methods: the use of metal protector anodes (galvanic protector method) and the use of external direct current sources, the minus of which is connected to the pipe, and the plus is connected to anode grounding (electrical method).
Rice. 1. Operating principle of cathodic protection
Galvanic tread protection against corrosion
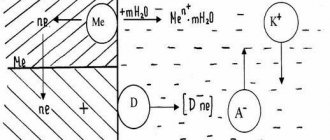
The most obvious way to carry out electrochemical protection of a metal structure that has direct contact with an electrolytic medium is the galvanic protection method, which is based on the fact that different metals in the electrolyte have different electrode potentials. Thus, if you form a galvanic couple from two metals and place them in an electrolyte, then the metal with a more negative potential will become an anode-protector and will be destroyed, protecting the metal with a less negative potential. Protectors essentially serve as portable sources of electricity.
Magnesium, aluminum and zinc are used as the main materials for the manufacture of protectors. From a comparison of the properties of magnesium, aluminum and zinc, it is clear that of the elements under consideration, magnesium has the greatest electromotive force. At the same time, one of the most important practical characteristics of protectors is the efficiency factor, which shows the proportion of the protector mass used to obtain useful electrical energy in the circuit. Efficiency protectors made from magnesium and magnesium alloys rarely exceed 50% in, in contrast to protectors based on Zn and Al with efficiency. 90% or more.
Rice. 2. Examples of magnesium protectors
Typically, protector installations are used for cathodic protection of pipelines that do not have electrical contacts with adjacent extended communications, individual sections of pipelines, as well as tanks, steel protective casings (cartridges), underground tanks and containers, steel supports and piles, and other concentrated objects.
At the same time, tread installations are very sensitive to errors in their placement and configuration. Incorrect selection or placement of tread units leads to a sharp decrease in their effectiveness.
Cathodic corrosion protection
The most common method of electrochemical protection against corrosion of underground metal structures is cathodic protection, carried out by cathodic polarization of the protected metal surface. In practice, this is realized by connecting the protected pipeline to the negative pole of an external direct current source, called a cathodic protection station. The positive pole of the source is connected by a cable to an external additional electrode made of metal, graphite or conductive rubber. This external electrode is placed in the same corrosive environment as the object being protected, in the case of underground field pipelines, in the soil. Thus, a closed electrical circuit is formed: additional external electrode - soil electrolyte - pipeline - cathode cable - DC source - anode cable. As part of this electrical circuit, the pipeline is the cathode, and an additional external electrode connected to the positive pole of the direct current source becomes the anode. This electrode is called anode grounding. The negatively charged pole of the current source connected to the pipeline, in the presence of external anodic grounding, cathodically polarizes the pipeline, while the potential of the anode and cathode sections is practically equalized.
Thus, the cathodic protection system consists of a protected structure, a direct current source (cathodic protection station), anode grounding, connecting anode and cathode lines, the surrounding electrically conductive medium (soil), as well as elements of the monitoring system - control and measuring points.
Drainage corrosion protection
Drainage protection of pipelines from corrosion by stray currents is carried out by directed drainage of these currents to a source or to the ground. Installation of drainage protection can be of several types: earthen, direct, polarized and reinforced drainage.
Rice. 3. Drainage protection station
Earth drainage is carried out by grounding pipelines with additional electrodes in the places of their anode zones, direct drainage is carried out by creating an electrical jumper between the pipeline and the negative pole of a source of stray currents, for example, the rail network of an electrified railway. Polarized drainage, unlike direct drainage, has only one-way conductivity, so when a positive potential appears on the rails, the drainage is automatically turned off. In enhanced drainage, a current converter is additionally included in the circuit, allowing the drainage current to be increased.
PS A review of technical solutions for ECP of other metal structures and structures can be read here .
Do you want to learn more about corrosion of metal structures and anti-corrosion protection methods?
Download our specialized training and reference application “Corrosion Protection”
Cathode polarization technology
In this case, the so-called superimposed current is used. An external conductor (often) or a current source (rarely) is used to apply it to a metal object. Upon contact with an electrically active particle, the following happens: the particle, under the influence of electrical attraction forces, moves to a protective element with a negative charge, where “recycling” of these particles occurs.
The consequences of such “disposal” are obvious - the protective element itself becomes corroded over time and becomes unusable. Therefore, this technology is often called the sacrificial electrode method (instead of our part, the “sacrificial electrode” rusts).
In addition to current and voltage, when working with cathodic polarization, one more important parameter must be taken into account - the ohmic voltage. In a technical sense, this parameter reflects the fact that as an electrical charge flows over time, the current voltage in the circuit drops. The drop itself occurs due to the fact that the cathode current flows along a circuit with a lower charge. If the circuit is assembled correctly, this indicator is quite small - thanks to this, the same current of the same power will always be maintained in the circuit.
Cathodic protection of gas pipelines against corrosion
This method consists of connecting the positive pole of the DC generator to the anode ground conductor.
From it, currents enter the soil, entering the pipeline through damaged areas of insulation. They are directed through the pipe to the place where the conductor is connected, and then to the negative edge of the source. If there is a sufficient voltage level, the entire working part of the gas pipeline becomes negative cathode. This makes it possible to prevent the formation of active corrosion. In this case, the grounding (waste metal) becomes the anode section. As a result, the pipe is negatively potentiated in relation to the ground.
Technology for creating protection stations
Another technology for creating cathodic protection is connecting the element to external current sources. In most cases, for these purposes, special cathodic protection stations (CPS) are built, which consist of several elements - the main current source, anode grounding, various cables and wires connecting individual structural elements and auxiliary points with mechanical or computer control, which allow you to control the parameters .
Most often, this technology is used for objects located near power lines - these can be pipelines, various factory buildings, and so on. VCS can operate in multi-threaded mode - in this case they will serve several protection systems at once. On pipes, a practice has become widespread in which several separate blocks are placed on the pipes to distribute the current more efficiently. The thing is that in the case of long pipelines, in the places where the pipes are connected to current sources, special points with an increased level of electric field voltage are formed - because of this, damage to the pipes can occur. The use of such blocks allows electricity to be distributed evenly throughout the entire protective circuit.
Automation
Checkpoints can operate both manually and automatically:
- In the case of manual control, the change in voltage parameters is regulated by the operator. At the physical level, regulation is carried out by switching the operation of the transformer. The operation of the winding is regulated, which allows you to change the parameters of the electric current.
- In the case of automatic control, the change in voltage parameters is regulated by the device itself based on the parameters that were once set by the operator. At the physical level, control is carried out using special semiconductor thyristors. They turn on or off when the electric current parameters deviate from the specified parameters.
Electrochemical protection (ECP)
Anti-corrosion protection products
- Corrosion of underground pipelines and protection against it
- Cathodic protection installations
- Drainage protection installations
- Galvanic protection installations
- Installations with extended or distributed anodes.
1. Corrosion of underground pipelines and protection against it
Corrosion of underground pipelines is one of the main reasons for their depressurization due to the formation of cavities, cracks and ruptures. Corrosion of metals, i.e. their oxidation is the transition of metal atoms from a free state to a chemically bound, ionic state. In this case, the metal atoms lose their electrons, and the oxidizing agents accept them. On an underground pipeline, due to the heterogeneity of the pipe metal and due to the heterogeneity of the soil (both in physical properties and chemical composition), areas with different electrode potentials appear, which causes the formation of galvanic corrosion. The most important types of corrosion are: superficial (solid over the entire surface), local in the form of shells, pitting, crevice and fatigue corrosion cracking. The last two types of corrosion pose the greatest danger to underground pipelines. Surface corrosion only rarely causes damage, while pitting corrosion causes the greatest number of damage. The corrosion situation in which a metal pipeline is located in the ground depends on a large number of factors related to soil and climatic conditions, route characteristics, and operating conditions. These factors include:
- soil moisture,
- chemical composition of the soil,
- acidity of the ground electrolyte,
- soil structure,
- temperature of transported gas
The most powerful negative manifestation of stray currents in the ground, caused by electrified DC rail transport, is electrocorrosive destruction of pipelines. The intensity of stray currents and their impact on underground pipelines depends on factors such as:
- rail-to-ground contact resistance;
- longitudinal resistance of running rails;
- distance between traction substations;
- current consumption by electric trains;
- number and cross-section of suction lines;
- electrical resistivity of soil;
- distance and location of the pipeline relative to the path;
- transition and longitudinal resistance of the pipeline.
It should be noted that stray currents in cathode zones have a protective effect on the structure, therefore, in such places, cathodic protection of the pipeline can be carried out without large capital costs.
Methods for protecting underground metal pipelines from corrosion are divided into passive and active.
The passive method of corrosion protection involves creating an impenetrable barrier between the metal of the pipeline and the surrounding soil. This is achieved by applying special protective coatings to the pipe (bitumen, coal tar pitch, polymer tapes, epoxy resins, etc.).
In practice, it is not possible to achieve complete continuity of the insulating coating. Different types of coating have different diffusion permeability and therefore provide different insulation of the pipe from the environment. During construction and operation, cracks, scuffs, dents and other defects appear in the insulating coating. The most dangerous are through damage to the protective coating, where, in practice, ground corrosion occurs.
Since the passive method does not allow complete protection of the pipeline from corrosion, active protection is simultaneously applied, associated with the control of electrochemical processes occurring at the boundary of the pipe metal and the ground electrolyte. This type of protection is called comprehensive protection.
The active method of corrosion protection is carried out by cathodic polarization and is based on reducing the rate of dissolution of the metal as its corrosion potential shifts to an area of more negative values than the natural potential. It was experimentally established that the value of the cathodic protection potential of steel is minus 0.85 Volts relative to the copper sulfate reference electrode. Since the natural potential of steel in the ground is approximately -0.55...-0.6 Volts, to implement cathodic protection it is necessary to shift the corrosion potential by 0.25...0.30 Volts in the negative direction.
By applying an electric current between the metal surface of the pipe and the ground, it is necessary to achieve a reduction in the potential in defective areas of the pipe insulation to a value below the protective potential criterion of -0.9 V. As a result, the corrosion rate is significantly reduced.
2. Cathodic protection installations Cathodic protection of pipelines can be carried out using two methods:
- the use of magnesium sacrificial protector anodes (galvanic method);
- using external direct current sources, the minus of which is connected to the pipe, and the plus to anode grounding (electrical method).
The galvanic method is based on the fact that different metals in the electrolyte have different electrode potentials. If you form a galvanic couple from two metals and place them in an electrolyte, the metal with a more negative potential will become the anode and will be destroyed, thereby protecting the metal with a less negative potential. In practice, protectors made of magnesium, aluminum and zinc alloys are used as sacrificial galvanic anodes.
The use of cathodic protection using protectors is effective only in low-resistivity soils (up to 50 Ohm-m). In high-resistivity soils, this method does not provide the necessary protection. Cathodic protection by external current sources is more complex and labor-intensive, but it depends little on the resistivity of the soil and has an unlimited energy resource.
As a rule, converters of various designs powered from an alternating current network are used as direct current sources. The converters allow you to regulate the protective current over a wide range, ensuring pipeline protection in any conditions.
0.4 overhead lines are used as power sources for cathodic protection installations; 6; 10 kV. The protective current applied to the pipeline from the converter and creating a “pipe-ground” potential difference is distributed unevenly along the length of the pipeline. Therefore, the maximum absolute value of this difference is located at the point of connection of the current source (drainage point). As you move away from this point, the pipe-ground potential difference decreases. Excessively increasing the potential difference negatively affects the adhesion of the coating and can cause hydrogenation of the pipe metal, which can cause hydrogen cracking. Cathodic protection is one of the methods of combating metal corrosion in aggressive chemical environments. It is based on transferring a metal from an active state to a passive state and maintaining this state using an external cathode current. To protect underground pipelines from corrosion, cathodic protection stations (CPS) are built along their route. The VCS includes a direct current source (protective installation), anode grounding, a control and measuring point, connecting wires and cables. Depending on the conditions, protective installations can be powered from an alternating current network 0.4; 6 or 10 kV or from autonomous sources. When protecting multi-line pipelines laid in one corridor, several installations can be installed and several anode groundings can be constructed. However, taking into account that during interruptions in the operation of the protection system, due to the difference in natural potentials of the pipes connected by a blind jumper, powerful galvanic couples are formed, leading to intense corrosion, the connection of the pipes to the installation must be carried out through special joint protection units. These blocks not only disconnect the pipes from each other, but also allow you to set the optimal potential on each pipe. Converters powered by a 220 V industrial frequency network are mainly used as direct current sources for cathodic protection in VSCs. The output voltage of the converter is adjusted manually, by switching the taps of the transformer winding, or automatically, using controlled valves (thyristors). If cathodic protection installations operate under time-varying conditions, which may be caused by the influence of stray currents, changes in soil resistivity or other factors, then it is advisable to provide converters with automatic control of the output voltage. Automatic regulation can be carried out according to the potential of the protected structure (potentiostat converters) or according to the protection current (galvanostat converters).
3. Drainage protection installations
Electric drainage is the simplest type of active protection that does not require a current source, since the pipeline is electrically connected to the traction rails of the source of stray currents. The source of the protective current is the pipeline-rail potential difference, which arises as a result of the operation of electrified railway transport and the presence of a field of stray currents. The flow of drainage current creates the required potential shift in the underground pipeline. As a rule, fuses are used as a protective device, but maximum load circuit breakers with reset are also used, that is, they restore the drainage circuit after the current dangerous for the installation elements subsides. As a polarized element, valve blocks assembled from several avalanche silicon diodes connected in parallel are used. The current in the drainage circuit is regulated by changing the resistance in this circuit by switching active resistors. If the use of polarized electric drains is ineffective, then reinforced (forced) electric drains are used, which are a cathodic protection installation, the rails of an electrified railway are used as an anode grounding electrode. The current of forced drainage operating in cathodic protection mode should not exceed 100A, and its use should not lead to the appearance of positive rail potentials relative to the ground in order to prevent corrosion of rails and rail fastenings, as well as structures attached to them.
Electrical drainage protection can be connected to the rail network directly only to the middle points of track choke transformers through two to a third choke points. More frequent connections are allowed if a special protective device is included in the drainage circuit. A choke can be used as such a device, the total input resistance of which to the signal current of the main railway signaling system with a frequency of 50 Hz is at least 5 Ohms.
4. Galvanic protection installations
Galvanic protection installations (protector installations) are used for cathodic protection of underground metal structures in cases where the use of installations powered by external current sources is not economically feasible: the absence of power lines, the short length of the facility, etc.
Typically, protector installations are used for cathodic protection of the following underground structures:
- tanks and pipelines that do not have electrical contacts with adjacent extended communications;
- individual sections of pipelines that are not provided with a sufficient level of protection from converters;
- sections of pipelines electrically isolated from the main line by insulating connections;
- steel protective casings (cartridges), underground tanks and containers, steel supports and piles and other concentrated objects;
- the linear part of the main pipelines under construction before the commissioning of permanent cathodic protection installations.
Sufficiently effective protection with protective installations can be carried out in soils with a specific electrical resistivity of no more than 50 Ohms.
5. Installations with extended or distributed anodes.
As already noted, when using a traditional cathodic protection scheme, the distribution of the protective potential along the pipeline is uneven. The uneven distribution of the protective potential leads to both excessive protection near the drainage point, i.e. to unproductive energy consumption and to a reduction in the protective zone of the installation. This disadvantage can be avoided by using a circuit with extended or distributed anodes. The ECP technological scheme with distributed anodes makes it possible to increase the length of the protective zone compared to the cathodic protection scheme with concentrated anodes, and also ensures a more uniform distribution of the protective potential. When using the ZHZ technological scheme with distributed anodes, various layouts of anode grounding can be used. The simplest is the scheme with anode groundings evenly installed along the gas pipeline. Adjustment of the protective potential is carried out by changing the anodic grounding current using an adjusting resistance or any other device that ensures a change in the current within the required limits. In the case of grounding from several grounding electrodes, the protective current can be adjusted by changing the number of connected grounding electrodes. In general, the ground electrodes closest to the converter should have a higher contact resistance. Protective protection Electrochemical protection using protectors is based on the fact that due to the potential difference between the protector and the protected metal in an electrolyte environment, the metal is restored and the protector body dissolves. Since the bulk of metal structures in the world are made of iron, metals with a more negative electrode potential than iron can be used as a protector. There are three of them - zinc, aluminum and magnesium. The main difference between magnesium protectors is the largest potential difference between magnesium and steel, which has a beneficial effect on the radius of protective action, which ranges from 10 to 200 m, which allows the use of fewer magnesium protectors than zinc and aluminum. In addition, magnesium and magnesium alloys, unlike zinc and aluminum, do not have polarization, accompanied by a decrease in current output. This feature determines the main use of magnesium protectors for the protection of underground pipelines in soils with high resistivity
Features of cathodic protection of pipes
Corrosion in pipelines usually occurs due to various defects and damage to the pipes - ruptures, cracking, cracks, and so on. Due to corrosion, the sealing of pipes is compromised, which can lead to complete or partial failure of the pipeline. This problem is especially acute for underground pipelines. When pipes are placed underground, areas with different electrical potentials are created. This is due to the heterogeneity of the soil and the presence of various debris of inorganic origin in the soil. If there is a serious potential difference, negatively charged ions in the ground begin to react with the metal. This leads to corrosion, which quickly destroys the pipeline.
Electric potential
Cathodic protection of pipelines against corrosion is carried out according to two standard schemes. Using cathodic polarization and by creating external stations. Pipeline protection should be aimed primarily at reducing the rate of destruction of the pipe material. This is done by reducing the electrical potential of the pipe in comparison with the electrical potential of the soil:
- The electrical potential of most modern pipes is approximately 0.8-0.9 volts.
- It has been experimentally shown that the main rocks of the soil have a potential of approximately 0.5-0.6 volts.
To equalize the electrical potentials, it is necessary to reduce the potential of the pipes by only 0.3-0.4 volts. This allows you to almost completely stop the appearance of rust. If the work is carried out correctly, the rate of natural rusting will be less than 1 mm per year.
Choosing a method
The technology for creating external protection stations is suitable for pipes. In this case, overhead power lines with voltages from 500 to 10,000 volts are used as power sources. The higher the voltage, the more pipes can be serviced. Sometimes there are no such lines in a particular area. In this case, it makes sense to install various generators.
External station technology has one major drawback. To create protection, labor-intensive and complex work will have to be carried out. This significantly increases the cost of creating a pipeline. When working with high voltage, excess electrical voltage may be created at the point of electricity supply - this can cause hydrogen cracking of pipes, so when carrying out installation work, electrical wiring must be done carefully.
Instead of the technology of protective stations, you can use the technique of using galvanic anodes to create a polarization effect. This technology is suitable for soils with low resistivity (up to 50 Ohms per 1 sq. m). If the soil resistivity is very high, then the technology of using galvanic anodes is practically useless due to its low efficiency.
Description of technology
Cathodic corrosion protection is carried out using a direct electric current applied to the workpiece, and makes the workpiece potential negative. Rectifiers are often used for this purpose.
The object that is connected to the source of electric current is considered the “minus”, that is, the cathode, and the connected ground is the anode, that is, the “plus”. The main condition is the presence of a good electrically conductive environment. For underground pipes, this is soil.
When implementing this technology, a difference in electric current potential must be maintained between the soil (the electrically conductive medium) and the object being processed. The value of this indicator can be determined using a high-resistance voltmeter.
Features of effective work
Corrosion is often the culprit of pipeline depressurization. Due to damage to the metal structure, cracks, cavities and ruptures form on the structure. This problem is extremely relevant for underground pipelines, because they are constantly in contact with groundwater.
In this situation, the cathodic technique allows to minimize the process of dissolution and oxidation of the metal alloy by changing the initial corrosion potential.
Practical test results suggest that the polarization potential of metal alloys using cathodic techniques slows down corrosion.
In order to achieve effective protection, it is necessary, using a direct electric current, to reduce the cathodic potential of the material that was used to create the pipeline. In this situation, the rate of metal corrosion will not exceed ten micrometers per year.
In addition, cathodic protection is the best solution for protecting underground pipelines from the influence of stray electric currents. Stray currents are an electric charge that penetrates the soil during the operation of a lightning rod, the movement of electric trains, etc.
To provide anti-corrosion protection, power lines or portable generators operating on diesel fuel or gas can be used.
Features of cathodic protection of cars
Corrosion on cars often appears suddenly. The speed of its spread is very high, since the car has a large number of moving parts. During operation, various small cracks and dents may form in such elements. This significantly increases the risk of corrosion. Cathodic protection of a car against corrosion is usually carried out by redistributing the electrical potential.
Usually special electronic modules are used, which are compact in size and mounted inside the car. Installation of such blocks takes no more than 20 minutes.
Additional processing
It is also worth noting that the cathodic protection method is usually combined with other techniques:
- All main parts of the car are coated with special paints and mastics. They create a protective layer on the metal surface. This layer is electrically neutral. Therefore, upon contact with electrically active substances or ions, rusting does not occur.
- Some vehicle components may be coated with protective cathode plates, which also minimize the risk of rust. Plates are usually used to cover the moving parts that are most likely to crack and become damaged. This is the bottom of the car, rear wheel arches, headlights, interior door surfaces, and so on.
Electrical protection methods
Steel gas pipelines and tanks laid in the ground are subject to electrical protection in all anodic and alternating zones, regardless of the corrosive activity of the soil. Electrical protection methods can be divided into two main groups:
• removal and neutralization of stray currents;
• protection outside the zone of stray currents.
With the help of electrical protective installations on gas pipelines, anodic and alternating zones are eliminated and protective (negative) potentials are created. Cathodic polarization of metal underground structures must be carried out so that the polarization protective potentials created on their entire surface (in absolute value) are not less than 0.55 V and not more than 0.80 V in relation to the non-polarizing hydrogen electrode, and also not less than -0 .85 V and no more than -1.15 V - to copper sulfate in any environment. The potential of the non-polarizing copper sulfate electrode relative to the standard electrode is taken to be 0.3 V.
Polarization potentials are measured according to the methodology given in GOST 9.602-2005 (Appendix P). Cathodic polarization of underground gas pipelines must be carried out in such a way as to exclude its harmful influence on neighboring metal structures:
• reduction (in absolute value) of the minimum or increase in the maximum protective potential on adjacent metal structures with cathodic polarization by more than 0.1 V;
• the risk of electrical corrosion on adjacent underground metal structures that previously did not require protection.
To protect gas pipelines from corrosion by stray currents, drains, cathode stations, protectors, insulating flanges and inserts, as well as jumpers to adjacent metal underground structures can be used. The choice of one or another method of protection depends on specific conditions and in most cases is determined by experimental comparison of the effectiveness of their action. In cases where one of the protection methods fails to provide protective potentials in all sections of the protected gas pipelines, a combination of several protection methods is used.
Electrical drainage is a method of protection that involves removing stray currents from the anode zone of the protected structure to their source. Drainage is the cheapest protection, creating a large protection zone (up to 5 km). To protect metal underground structures, three types of drainage are applicable: direct, polarized and reinforced. For many reasons, the last two are most often used.
In the practice of autonomous gas supply, drainage has very limited use, since it does not provide the proper level of protection. In addition, it is easier to provide a rational gas pipeline route, excluding the influence of stray currents from electric rail vehicles, even at the design stage.
Cathodic protection. The principle of this type of protection is the cathodic polarization of the protected metal surface and giving it a negative potential relative to the environment using a direct current source.
The protected structure plays the role of an anode. The negative pole of the current source is connected to the gas pipeline (reservoir), and the positive pole is connected to the ground (anode). At the same time, the anodic grounding is gradually destroyed, protecting the gas pipeline. This type is applicable both for protection against corrosion by stray currents and soil.
The effectiveness of cathodic protection depends on the condition of the insulating coatings. With good insulation, energy consumption is reduced and the length of protected sections of metal structures increases. The average electrical energy consumption per year per cathodic protection station is about 500 kWh.
The schematic diagram of cathodic protection is shown in Fig. 6.2: the current from the positive pole of the source passes through the connecting cable and anodic grounding into the ground. From the soil, through defective places in the insulation, the current penetrates into the gas pipeline and is directed through the drainage cable to the negative pole of the source, creating a closed circuit through which the current flows from the anode through the ground to the gas pipeline and further along it to the negative pole of the source.
In this case, the anode is gradually destroyed, which protects the structure from corrosion under the influence of its cathodic polarization. Insulated cables with a cross section of 25-77 mm2 (depending on the power of the station) are used as connecting wires.
Table 6.5. Polarization protective potentials of the metal of a structure relative to a saturated copper-sulfate reference electrode
| Metal structures | Protective potential value, V | |
| minimum Emin | maximum Emax | |
| Steel | — 0,85 | -1,15 |
| Lead | -0,70 | -1,30 |
| Aluminum | — 0,85 | -1,40 |
For cathodic protection, the following gas pipeline-ground potentials are recommended, V:
- maximum permissible from soil corrosion - 1.2-1.5;
- from corrosion by stray currents - 2.5-9.0;
- minimum protective - 0.85 (relative to copper sulfate electrode).
To protect gas pipelines and tank farms, cathode stations of various capacities are used.
Cathode installations are most appropriate for protection against soil corrosion and are less effective when protecting against stray currents. The operation of cathodic protection installations is accompanied by increased consumption of electrical energy.
Sacrificial protection is a type of cathodic protection that is widely used. The necessary protective current is generated by a galvanic cell, the role of the cathode is played by the metal of the protected structure, the anode is played by a metal with potentials more negative than that of the protected metal, and the electrolyte is the soil surrounding the gas pipeline and the protector.
The protector protection installation consists of a protector (or a group of them), an activator or filler, connecting wires and a terminal box (in the case of a group installation of protectors).
Protective protection (polarized anodic protectors) is used to protect underground structures from corrosion released by stray currents in the anodic and alternating zones, when the strength of stray currents can be compensated by the protector current and the required protective potential is provided in accordance with the requirements of GOST 9.602-2005.
Table 6.6. Areas of application of protectors depending on the corrosive activity of the soil
| Electrical resistivity, Ohm*m | Corrosive activity of soil | Protectors used |
| Up to 5 | Very high | Magnesium and zinc protectors weighing 20 kg (magnesium protectors are not used at pH4) |
| 5-10 | High | Magnesium and zinc protectors weighing 10-20 kg (magnesium protectors are not used at pH4) |
| 10-20 | Increased | Magnesium protectors weighing 10 kg |
| 20-50 | Average | Magnesium protectors weighing 5 kg |
Protective protection consists of attaching to the protected structure metal plates or rods (protectors) that have a lower electrical potential than the metal of the structure (Fig. 6.3). At the same time, the total metal losses do not decrease, but, on the contrary, increase. The advantage of this method of protection is that corrosion is transferred from a more valuable and hard-to-reach structure of the structure (gas pipeline) to a cheaper and easily renewable one (the tread).
The key characteristic of a tread is its surface area. Industrial protectors are made from magnesium or aluminum alloys. During storage in a warehouse and during transportation, the protector is additionally packaged in a paper bag, which is removed before installing the protector in the ground.
The effectiveness of tread protection largely depends on the correct choice of tread material and the environment in which the latter is located. The most commonly used are magnesium, aluminum and zinc protectors and their alloys. Protectors are widely used to protect underground gas pipelines and tanks with liquefied hydrocarbon gases from soil corrosion. To protect steel tanks of liquefied gases from corrosion, it is allowed to provide protectors as the main grounding conductors for protection against direct lightning strikes. In this case, one should be guided by the requirements of RD 34.21.122-87.
Table 6.7. Characteristics of tread alloys
| Alloy grade | Potential at copper sulfate reference electrode, V | Theoretical current output, A*h/kg | Efficiency, % |
| Ml 16 | -1,6 | 2200 | 52 |
| Ml 16 pch | -1,6 | 2200 | 60 |
| Ml 16 hh | -1,6 | 2200 | 62 |
| ml 4 hf | — 1,55 | 2200 | 64 |
| MP1 | 1,55 | 2200 | 65 |
| CPU1 | — 1,1-1,15 | 820 | 95 |
| CPU2 | — 1,1-1,15 | 820 | 95 |
| AP1 | -1,04 | 2880 | 75 |
| AP2 | -0,94 | 2960 | 70 |
| AP3 | — 1,04 | 2880 | 85 |
| AP4 | -1,14 | 2880 | 85 |
| AP5 | — 1,02 | 2700 | 70 |
Long-term non-polarizing copper sulfate comparison electrodes are used for measuring the potential difference between underground structures and the ground, determining the effectiveness of anti-corrosion protection of underground metal structures and ensuring the operation of cathodic protection rectifiers in the mode of automatically maintaining the measured potential difference and for measuring the polarization potential of a protected structure with portable instruments.
Table 6.8. Performance characteristics of protectors
| Alloy grade | Stationary potential in the activator (MSE), mV | Practical current output, Ah/kg |
| Ml16 | 1590 | 1100 |
| Ml16ach | 1620 | 1400 |
Electrodes of the ENES type (TU 47 3994-002-10244915-95) are installed permanently in the ground to a depth of 0.8 to 3 m with conductors leading out to a control point or carpet, and can also be used as portable ones.
Table 6.9. Chemical composition of magnesium and zinc tread alloys
| Alloy grade | Main components, % | Impurities, no more, % | |||||||
| Mg | Al | Zn | Mn | Fe | Cu | Ni | Si | Ti | |
| Ml16 | rest | 7,5-9,0 | 2,0-3,0 | 0,15-0,50 | 0,03 | 0,15 | 0,01 | 0,2 | — |
| Ml16pch | 0,005 | 0,01 | 0,001 | 0,06 | — | ||||
| Ml16vch | 0,003 | 0,003 | 0,001 | 0,04 | — | ||||
| Ml4vch | 5,0-7,0 | 0,003 | 0,004 | 0,001 | 0,05 | — | |||
| MP1 | 2,0-4,0 | 0,02-0,50 | 0,003 | 0,004 | 0,001 | 0,04 | 0,04 | ||
| CPU1 | — | 0,4-0,6 | rest | — | 0,001 | 0,001 | — | — | — |
| CPU2 | 0,1-0,3 | 0,5-0,7 | 0,1-0,3 | 0,004 | 0,001 | — | — | — | |
Operation of ENES electrodes is carried out in the temperature range -40…+45°C. ENES-1 electrodes are produced in a hermetically sealed design using ion-exchange membranes, through which contact with the ground is ensured without loss of electrolyte. The ion exchange membrane is protected from damage by a lattice cover. A potential sensor with a removable attachment is attached to the electrode body, made of glass-filled polyamide. The electrodes work reliably with cathodic protection stations having an input resistance of the measuring circuit of 10 kOhm and higher.
Insulating flange connections (IFS) are an additional means of protecting gas pipelines from corrosion, used in conjunction with electrochemical protection devices.
Protection of gas pipelines using IFS is that the gas pipeline is divided into separate sections, thus reducing its conductivity (and the strength of the current flowing through the gas pipeline). By dividing the gas pipeline into sections (sections), the solution to the issue of protecting them is simplified. Typically, IFS (gaskets between flanges made of rubber or ebonite) and inserts (made of polyethylene pipes) are used to cut off various underground structures (gas pipeline and heat pipeline in a boiler room, gas pipeline and water supply to a house, etc.) from each other, as well as to disconnect buildings according to their ownership.
Installation of IFS on gas pipelines is most often provided on the risers of incoming gas pipelines to consumers, where electrical contact of the gas pipeline with grounded structures and communications is possible; at underground and overwater passages of gas pipelines through obstacles (in vertical sections), as well as at the inputs (and outputs) of gas pipelines to gas distribution stations, gas distribution centers, gas distribution centers. On each side of the IFS, control conductors are installed with output to the surface.
Electrical jumpers. This method of protection is used in cases where one structure has a positive potential (anode zone), and the other has a negative potential (cathode zone), that is, their electrical connection by jumpers leads to the fact that negative potentials are established on both structures. Such jumpers are used to combine local and main (distant) gas pipelines, as well as when laying gas pipelines of different pressures, for example high and low, along the same street or in the same area. Jumpers are widely used for joint protection of various structures. Electrical jumpers between gas pipelines, made of strip steel, must have a very reinforced insulating coating.
- home
- Directory
- Protection of gas pipeline and tank equipment from corrosion
- Electrical protection methods
Electrochemical protection
Electrochemical protection refers to active methods of protection against external corrosion, which involve the creation of such an electric current in which the entire metal of the pipeline, despite the heterogeneity of its inclusions, becomes the cathode, and the anode is the metal additionally placed in the ground. There are two types of active protection of pipelines from external corrosion - sacrificial and cathodic .
Protective protection of pipelines
The fight against metal corrosion is relevant in the oil and gas industry (due to corrosion destruction of the bottoms of oil sludge tanks and field pipelines) and other areas of production activity with a high probability of man-made disasters. Tread protection of a pipeline against corrosion is based on stopping the corrosion of metals under the influence of direct electric current. Protective protection is applied simultaneously with protective paint and varnish coatings. This combination allows you to increase their service life and ensures uniform distribution of current over the surface of structures, which compensates for coating defects that arise during operation.
Cathodic protection of pipelines
Cathodic protection is a method of protecting structures by forced cathodic polarization using an external direct current source. The negative pole of the external current source is connected to the protected structure, which acts as a cathode. To form a current-closed circuit, the positive pole of the source is connected to an auxiliary electrode - the anode, which is located in the same environment (soil, water) as the protected object. Thus, cathodic protection consists in the fact that the protected object is negatively polarized and its potential shifts to a value at which the metal corrosion process is significantly or completely suppressed. Cathodic protection is an auxiliary type of protection, therefore cathodic protection is used in conjunction with insulating coatings applied to the outer surface of the protected structure. Otherwise, cathodic polarization of a bare pipeline to the minimum protective potential would require significant protective currents.
Electrochemical protection of pipelines
LLC "GKNT" implements an integrated approach when performing work on electrochemical protection of pipelines:
- Monitoring the corrosion state of underground metal structures and structures
- Installation and operation of installations for electrochemical protection
- Carrying out construction and installation work to organize electrochemical protection
- Carrying out commissioning works for electrochemical protection
- Inspection of electrochemical protection systems and issuance of a technical report
- Carrying out electrical measuring work in our own licensed laboratory
- Supply of equipment, instruments and materials for performing maintenance work on electrochemical protection installations
Installation of racks of control and measuring points
Control and measuring points are designed to indicate the location of underground pipeline routes and monitor their electrochemical protection. They are located on industrial sites of gas distribution stations, on linear parts of underground pipelines, at oil and gas production facilities, in underground oil and petroleum product storage facilities, in underground gas storage facilities, and other industrial facilities with underground metal structures. The control and measuring point consists of a rack and a terminal mounted on the rack. The stand, at the request of the customer, is made of polyvinyl chloride (PVC), metal or fiberglass. The materials used in the installation of racks of control and measuring points are specifically designed for their operation in all climatic zones in the open air. The stand is equipped with an anchor device that prevents the control and measuring point from being freely removed from the ground. The kit additionally includes a kilometer sign, which allows you to visually monitor the location of the pipeline route from the air.
Installation of cathode potential measurement and control systems
LLC "GKNT" installs systems for measuring and regulating cathode potential for the protection of underground structures. All main pipelines, underground wells and storage facilities are equipped with devices for cathodic corrosion protection. Electrochemical protection of pipelines is carried out, as a rule, from cathodic protection stations; sacrificial anodes are used only in the absence of a current source. Cathodic protection systems must regulate the cathodic potential by connecting the negative pole of a direct current source to the protected surface, while the positive pole is connected to specially installed anodes.
Installation of deep anode grounding conductors, creation of anode fields
Deep grounding electrodes are designed for operation in places of limited land allocation for the anode field, as well as for installation in places with low electrical conductivity of the surface soil layer and in geologically complex areas. Deep anode grounding electrodes are designed to protect above-ground and underground oil product tanks, main oil and gas pipelines, underground steel structures, wells, grounding of power lines and other metal structures that are in contact with soil and water. Anodic grounding electrodes are made on the basis of iron-silide alloys; they are resistant to anodic dissolution when working in aggressive alkaline or acidic soils, in fresh and brackish waters and are intended for use in any soil.
Installation of pipeline cathodic protection stations
LLC "GKNT" carries out installation of cathodic protection stations, which are very important in the operation of stationary oil and gas field structures, oil and gas pipelines, and pipelines on the continental shelf. Cathodic protection of underground structures is widespread. Most main pipelines, underground storage facilities and wells are equipped with cathodic protection devices in combination with protective paint coatings.
Electrochemical protection of pipelines at crossings over water barriers, roads and railways
Electrochemical corrosion protection is a set of measures to reduce the electrical potential of pipes and soil. The creation of electrochemical protection of pipelines is regulated by the requirements of SNiP 2.05.06-85. At pipeline crossings under railways and highways, the sections of pipelines that are adjacent to them must have casings and a reinforced type of protective coating. Electrochemical protection of casings at crossings over water barriers and under roads and railways must be done simultaneously with the protection of the main pipeline itself. When commissioning a main pipeline and during its operation, electrical contact between the pipeline and the casing should be regularly monitored and, if detected, it should be eliminated.