- Causes of corrosion
- Types of corrosion
- Solid
- Local
The word corrosion comes from the Latin corrodere. It literally means “to corrode.” The most common type of corrosion is metal. However, there are cases when products made from other materials also suffer from corrosion. Stones, plastic and even wood are susceptible to it. Today, more and more often, people are faced with the problem of corrosion of architectural monuments made of marble and other materials. From this we can conclude that a process such as corrosion means destruction under the influence of the environment
Causes of metal corrosion
Most metals are susceptible to corrosion. This process is their oxidation. It leads to their decomposition into oxides. In common parlance, corrosion is called rust. It is a finely ground light brown powder. On many types of metals, during the oxidation process, a special composition appears in the form of an oxide film bonded to them. It has a dense structure, due to which oxygen from the air and water cannot penetrate into the deep layers of metals for their further destruction.
Aluminum belongs to the category of very active metals. From a theoretical point of view, when it comes into contact with air or water, it should easily split. However, during corrosion, a special film is formed on it, which compacts its structure and makes the process of rust formation almost impossible.
Chemical coatings
Refers to methods of temporary anti-corrosion protection of steel, for example, during plastic deformation at elevated temperatures. The most widely used technologies are phosphating and oxalation.
When phosphating, the surface is covered with a continuous layer of phosphate salts of iron and manganese, and when oxalating, it is covered with water-soluble salts of oxalic acid. Phosphating is used for processing unalloyed steels, oxalation – for alloyed ones. The coating adheres firmly to the surface, helping to reduce friction and reduce tool wear. After stamping is completed, the coating is removed.
Table 1. Metal compatibility
| Metals for which data are presented in the table on their susceptibility to corrosion | Ratio of metal area to other metals table | Magnesium | Zinc | Aluminum | Cadmium | Lead | Tin | Copper | |||||
| Magnesium | Low | WITH | WITH | WITH | WITH | WITH | WITH | ||||||
| High | U | U | U | WITH | WITH | ||||||||
| Zinc | Low | U | U | U | WITH | WITH | WITH | ||||||
| High | N | N | N | N | N | N | |||||||
| Aluminum | Low | U | N | N | WITH | WITH | |||||||
| High | N | U | N | WITH | WITH | WITH | |||||||
| Cadmium | Low | N | N | N | WITH | WITH | WITH | ||||||
| High | U | N | N | N | N | N | |||||||
| Carbon steel | Low | N | N | N | N | WITH | WITH | WITH | |||||
| High | N | N | N | N | N | N | N | ||||||
| Low alloy steel | Low | N | N | N | N | WITH | WITH | WITH | |||||
| High | N | N | N | N | N | N | N | ||||||
| Cast steel | Low | N | N | N | N | WITH | WITH | WITH | |||||
| High | N | N | N | N | N | N | |||||||
| Chrome steel | Low | N | N | N | N | U | U | WITH | |||||
| High | N | N | N | N | N | N | |||||||
| Lead | Low | N | N | N | N | N | N | ||||||
| High | N | N | N | N | N | ||||||||
| Tin | Low | N | N | N | N | N | |||||||
| High | N | N | N | N | N | ||||||||
| Copper | Low | N | N | N | N | U | WITH | ||||||
| High | N | N | N | N | N | U | |||||||
| Stainless steel | Low | N | N | N | N | N | N | ||||||
| High | N | N | N | N | U | U | N | ||||||
Column 1 of the table presents metals that are or are not subject to corrosion with the metals indicated in the remaining columns of the table and the proportion of the ratio of the areas of the metal indicated in column 1 to the metals in the remaining columns of the table. The short designation S, U, N in the table means:
| |||||||||||||
Appendix C. Metal structures. Corrosion protection requirements
Printable version Table C.1. Groups of paint and varnish coatings for the protection of metal structures
| Operating conditions of structures | Degree of aggressive environmental influence | Group of paint and varnish coating for steel structures according to table Ts.8, total thickness of paint and varnish coating, including primer, microns | ||||
| construction material | metal protective coating material | |||||
| carbon and low-alloy steel without metal protective coatings | galvanized steel class I or class no less than 275 | zinc coatings (hot and thermal diffusion galvanizing) | zinc and aluminum coatings (thermal spraying) | |||
| Inside heated and unheated buildings | Rooms with gases of group A or poorly soluble salts and dust | Slightly aggressive | I-80 | II-40 | Without paint coating | |
| Moderately aggressive | II-160 | II-160 | II-120 | II-120 | ||
| Rooms with gases of groups B C D or highly soluble (low-hygroscopic and hygroscopic) salts, aerosols and dust | Slightly aggressive | III-120 | III-60 | Without paint coating | ||
| Moderately aggressive | III-160 | III-160 | III-160 | III-160 | ||
| Highly aggressive | IV-240 | IV-240 | Do not apply | IV-240 | ||
| Outdoors and under canopies | Group A gases or poorly soluble salts and dust | Slightly aggressive | I-80 | II-40 | Without paint coating | |
| Moderately aggressive | II-160 | Do not apply | II-120 | II-120 | ||
| Gases of groups B, C, D or highly soluble (low-hygroscopic and hygroscopic) salts, aerosols and dust | Slightly aggressive | III-120 | III-60 | Without paint coating | ||
| Moderately aggressive | III-160 | Do not apply | III-120 | IV-240 | ||
| Highly aggressive | IV-200 | Do not apply | Do not apply | III-120 | ||
| In liquid media | Slightly aggressive | III-160 | Do not apply | III-160 | III-160 | |
| Moderately aggressive | IV-220 | Do not apply | IV-180 | IV-200 | ||
| Highly aggressive | IV-300-500 | Do not apply | Do not apply | IV-240 | ||
| Notes 1 On welded seams, the thickness of the coatings should be increased by 30 microns. 2 When choosing paint and varnish coatings, you should take into account the specific features of the operation of metal structures. Depending on the operating conditions, the paint and varnish coatings used must be resistant outdoors, under a canopy, and indoors - chemically resistant, heat-resistant, oil-resistant, water-resistant, acid-resistant, alkali-resistant, gasoline-resistant. | ||||||
Table C.2 - Methods of protecting steel chimneys
| Gas temperature, °C | Composition of gases | Relative humidity of gases, % | Possibility of condensation | Steel grades | Methods of protection against corrosion |
| St. 89 to 140 | By groups A and B | Up to 30 | Not formed | VSt3sp5 | Epoxy heat-resistant coatings1) |
| St. 140 to 250 | SO2, SO3, | St. 10 to 15 | Same | VSt3sp5 | Thermal spraying2) or silicone coatings1) |
| St. 69 to 160 | Same | St. 10 to 20 | Formed | 2X13, 3X13, 12Х18Н10Т | No protection |
| St. 69 to 160 | SO2, SO3, nitrogen oxides | St. 10 | Same | 0Х20Н28МДТ, 10Х17Н13М2Т, 12Х18Н10Т | Same |
| 1) According to table Ts.6, and for epoxy materials - only with short-term temperature increases above 100 ° C; the number of layers and thickness of the coating are assigned as for moderately aggressive environments in rooms with gases of groups B, C, D. 2) Aluminum with a layer thickness of 200-250 microns. | |||||
Table C.3 - Coating materials for corrosion protection of internal surfaces of steel tanks for liquid media
| The degree of aggressive influence of the liquid environment | Coating materials |
| Moderately aggressive | Gas-thermal aluminum coatings, paint and varnish, reinforced paint and varnish, liquid rubber, mastic, lining1), gumming |
| Highly aggressive | Gas-thermal aluminum coatings with subsequent application of paint and varnish coatings, reinforced paint and varnish coatings, sheet cladding, combined linings, rubber coatings |
| 1) Provided for paint or mastic coating in the presence of an abrasive environment or shock loads. | |
Table T.4 - Protection of steel ropes used outdoors
| Humidity zone according to SP 50.13330 | Degree of aggressive environmental influence | Rope construction | Tensile strength of wire for ropes, MPa | Group of zinc coated wires |
| Dry | Slightly aggressive | Any | Before 1764 | G1) or coolant2) |
| Normal | Same | Same | Before 1764 | Coolant2) |
| Dry, normal, wet | Moderately aggressive or highly aggressive | Closed design | External rope turns up to 1372, internal rope turns up to 1764 | Coolant with additional protection by paint coatings, lubricants or polymer films |
| 1) In the absence of constant monitoring of the condition of structures during operation, it is necessary to provide additional protection with paint and varnish coatings, lubricants or polymer films. 2) For layers of wire from the first to the penultimate, coating group Zh is allowed. | ||||
Table C.5 - Materials for welding steel structures in aggressive environments, corresponding to low-alloy steel grades
| Degree of aggressive environmental influence | steel grade | Welding material grades | ||
| welding wire | coated electrodes | |||
| submerged | in carbon dioxide | |||
| Slightly aggressive1) | 10HNDP, 10HDP | Sv-08Х1ДУ, Sv-10НМА, Sv-08ХМ | PPV-5k2), Sv-08ХG2SDYu | OZS-18 |
| 10HSND, 15HSND | Sv-10NMA, Sv-08XM | Sv-08HG2SDYu | OZS-24, AN-X7, VSN-3, E138-45N, E138-50N3) | |
| Moderately aggressive and highly aggressive | 10HSND, 15HSND | Sv-10NMA, Sv-08ХМ | Sv-08HG2SDYu | AN-X7, VSN-3, E138-45N, OZS-24, E138-50N3) |
| 10HNDP, 10HDP | Sv-08Х1ДУ, Sv-10НМА, Sv-08ХМ | Sv-08HG2SDYu | OZS-18 | |
| 09G2S, 10G2S1 | Sv-10G2, Sv-10GA, Sv-08GA | Sv-08G2S, Sv-08G2STs | SSSI 13/55 | |
| 18G2AFps, 16G2AF, 15G2AFDps, 14G2AF | — | Sv-08G2S, Sv-08G2STs | SSSI 13/65 | |
| 12GN2MFAYU, 12G2SMF | Sv-08HGN2MYU | Sv-10ХГ2СМА | Any type E70 | |
| 1) When designing structures without corrosion protection. 2) Without additional protection. 3) Only for steel grade 10HSND. Notes 1 Electrode coating for manual welding of structures made of steel grades 10ХСНД and 15ХСНИ should be selected in agreement with customers and installation organizations. 2 When designing welded joints, the use of welding materials not listed in Table Ts.5 may be provided, if the possibility of their use is confirmed in the manner established by the Legislation of the Russian Federation in the field of technical regulation. | ||||
Table C.6 - Methods of protection against corrosion of metal structures
| The degree of aggressive environmental influence on the structure | Constructions | ||
| carriers | fencing sheet assembly1)2) | ||
| carbon and low carbon steel | aluminum | made of galvanized steel with coating of class 1 or class not less than 275 | |
| Non-aggressive | Paint and varnish coatings of group I | No protection | Without protection2) on the room side when applying bitumen or paint and varnish coatings of groups II and III on the insulation side |
| Slightly aggressive | thermal diffusion zinc coatings ( t = 45-60 microns); hot zinc coatings ( t =60-100 microns); gas-thermal zinc coatings ( t =120-180 microns) or aluminum ( t =200-250 microns); paint coatings of groups I, II and III; insulating coatings (for structures in soil) | Same | a) paint coatings of groups II and III according to Table Ts.8, applied on lines for continuous painting of rolled metal (application of a bitumen coating on the insulation side is allowed); b) paint and varnish coatings of groups II and III according to Table C.7 (for structures located indoors, it is allowed to provide for the application of paint and varnish coatings 8-10 years after installation of the structures) |
| Moderately aggressive | thermal diffusion zinc coatings ( t = 45-60 microns) with overlapping paint coatings of groups II and III; hot zinc coatings ( t = 60-100 microns) with overlapping paint coatings of groups II and III; gas-thermal zinc or aluminum coatings ( t = 120-180 microns) with overlapping paint coatings of groups II, III and IV; paint coatings of groups II, III and IV; gas-thermal zinc coatings ( t =200-250 microns) or aluminum ( t =250-300 microns); insulating coatings together with electrochemical protection (for structures in soil)3); electrochemical protection in liquid media and bottom soils3); cladding with chemically resistant non-metallic materials | electrochemical anodic oxide coatings ( t = 15 µm); without protection2); chemical oxidation followed by application of paint and varnish coatings of groups II, III; paint and varnish coatings of group IV; the same, using a protective zinc-filled primer | Not allowed for use |
| Highly aggressive | gas-thermal aluminum coatings ( t = 200-250 microns) with overlapping paint coatings of group IV; insulating coatings together with electrochemical protection (for structures in soil)3); electrochemical protection (in liquid media)3); cladding with chemically resistant non-metallic materials; Group IV paint coatings | electrochemical anodic-oxidized coatings ( t = 15 microns) with overlapping paint coatings of group IV; Group IV paint coatings using a protective zinc-rich primer; the same, with preliminary chemical oxidation | Not allowed for use |
| 1) Does not apply to enclosing structures of three-layer metal panels. 2) In accordance with the requirements of table X.8. 3) Electrochemical protection is not provided for structural elements made of ropes and cables. Notes 1 The group and thickness of the paint coating are given in Table C.1. For non-aggressive environments, the thickness of the paint coating layer should be established according to regulatory documents. 2 In slightly aggressive, moderately aggressive and highly aggressive environments containing sulfur dioxide, hydrogen sulfide, nitrogen oxides of gas groups D* , C and D* , aluminum grades A7, AD1, AMts should be used for gas-thermal coatings; in other environments for gas-thermal and hot zinc coatings - zinc grades Tsch, Ts1, Ts2, Ts3.__________________ * The text of the document corresponds to the original. — Note from the database manufacturer. To protect against corrosion of steel structures exposed to liquid environments (moderately aggressive or highly aggressive), it is allowed to use thermally zinc coatings ( t = 80-120 µm) with overlapping aluminum coatings ( t = 120-170 µm). 3 Insulating coatings for structures in soil (bitumen, bitumen-rubber, bitumen-polymer, bitumen-mineral, ethylene, etc.) must meet the requirements of regulatory documents. | |||
Table C.7 - Groups of paint and varnish coatings for protecting steel and aluminum structures from corrosion
| Characteristics of paint and varnish material by film-forming type | Coating group | Index characterizing durability | Conditions for using coatings on steel and aluminum structures |
| Glypthal | I | — | Used for alkyd glyphthalic primer coatings on steel under enamels and group I paints |
| Alkyd-styrene | I | — | Used for primer coatings on steel under enamels of groups I, II |
| Epoxy ester | I | — | Used for primer coatings on steel under enamels of groups I, II |
| Pentaphthalic | I | a, an, p | Apply over group I primers |
| Nitrocellulose | I | a, an, p | Same |
| Alkyd-urethane | I | a, an, p | Same |
| Oily | I | a, an, p | |
| Bitumen-oil | I | a, an, p, t | Same as heat resistant without primer |
| Phenol-formaldehyde | II | — | Used for primer coatings on steel under perchlorovinyl, copolymer-vinylchloride and chlorinated rubber enamels of groups II, III. When pigmented with passivating pigments, it is used for primer coatings on galvanized steel and aluminum alloys |
| Polyvinyl butyral | II | — | Used as phosphating primers on steel and galvanized steel under primer coatings of groups I, II |
| Acrylic | II | a, an, p | Used as passivating primers on aluminum alloys, steel and galvanized steel under enamels of groups II, III. Acrylic enamels are applied over acrylic primers |
| Organosilicate | II, III | a, an, p | Can be applied without primer or over phosphating primer, alkyd, phenol-formaldehyde or organosilicate primers |
| Organosilicon | III | a, an, p, t | Apply over alkyd, phenol-formaldehyde or organosilicate primers, as oil-resistant and heat-resistant ones, applied without a primer |
| Chlorinated rubber | II, III | a, an, p, x | Chlorinated rubber enamels are applied over chlorinated rubber and acrylic primers |
| Polysiloxane | III | a, an, p, x | Applied over polysiloxane primers, and when combined also over epoxy primers |
| Polyurethane | III, IV | a, an, p, x | Can be applied over alkyd, phenol-formaldehyde, acrylic, epoxy and polyurethane primers |
| Polyurea | III, IV | X | Can be applied over one-component polyurethane primers or directly onto metal |
| Perchlorovinyl and copolymer-vinyl chloride | II, III, IV | a, an, p, x, xk, xsh | Applied over alkyd, phenol-formaldehyde, acrylic passivating and perchlorovinyl, copolymer-vinyl chloride primers |
| Epoxy | III, IV | a, an, p, x, hsh | Apply over epoxy primers |
| Protective zinc-filled on various film-forming materials (epoxy, polystyrene, polyurethane) | III | — | Used for priming coatings on steel under perchlorovinyl, copolymer-vinyl chloride, chlorinated rubber, polyurethane, epoxy enamels of groups III, IV, if it is necessary to ensure reliable and long-term protection of structures from corrosion |
| Designations: "a" - outdoors, "an" - the same, under a canopy, "p" - indoors, "x" - chemically resistant, "xk" - resistant in acid solutions, "khsh" - resistant in solutions alkalis, “t” - heat-resistant. | |||
Table C.7 (Changed edition, Amendment No. 1).
Table C.8 - Paint and varnish coatings for corrosion protection of thin galvanized sheet metal, applied on lines for continuous painting of coiled metal
| Characteristics of paint and varnish material by type of film-forming substance | Coating group | Short designation | Range of paint coating thicknesses depending on the index of operating conditions according to table X.9, including the primer layer, µm | |
| C11), C2 | C3 | |||
| Polyester thin layer | II | PE | 25-35 | — |
| Polyester, polyamide modified | II | PE (SP-PA) | 30-40 | 40-50 |
| Polyester thick layers | II | PE (HBP) | 30-40 | 40-50 |
| Polyester wear-resistant | II | PE (HDP) | 30-40 | 40-60 |
| Polyester wear-resistant, polyamide modified | II | PE (HDP-PA) | 30-40 | 40-60 |
| Polyester silicone | II | ML (SP-SI) | 30-40 | 40-60 |
| Polyurethane | III | UR (PUR) | 30-40 | 40-60 |
| Polyurethane modified with polyamide | III | UR (PUR-PA) | 30-40 | 40-60 |
| Polyfluoroethylene/ vinyl ester | III | FEVE | 30-40 | 40-60 |
| Polyvinylidene fluoride | III, IV | PVDF | 30-40 | 40-60 |
| Polyvinyl chloride plastisol | III | PLHV (PVC) | — | 100-220 |
| 1) For operating conditions with index C1, the thickness of the paint coating layer should be established according to regulatory documents. Note - The choice of grades of materials and thickness of protective and decorative paint coatings for additional protection against corrosion of galvanized steel is made taking into account the service life of the paint coating under specific operating conditions. The predicted service life of the coating should be established based on the results of accelerated climatic tests of coating samples. | ||||
Table C.8 (Changed edition, Amendment No. 1).
Table C.9. Options for protective coatings for steel tanks for acids, alkalis and liquid mineral fertilizers
| Protective coatings | Coverage schemes | Approximate coating thickness, mm |
| Paintwork | Paint and varnish coatings of group IV with the index “x”, “xk”, “xsh” according to table Ts.7, depending on operating conditions according to table Ts.1 | 0,16-0,50 |
| Reinforced paint and varnish | Fiberglass reinforced epoxy coatings | 1,0 |
| Polypropylene fabric-reinforced coatings based on polyester resins | 1,0 | |
| Liquid rubber compounds | Thiokol sealants for epoxy primers | 1,5-2,0 |
| Sealant based on divinylstyrene thermoplastic elastomer | 1,5-2,0 | |
| Mastic | Mastics based on epoxyfuran resins | 1,0-2,0 |
| Polymer putty based on epoxy compound | 1,0-2,0 | |
| Epoxy-shale compositions based on epoxy resins | 1,0-1,5 | |
| Leafy | Profiled polyethylene | 2,0-3,0 |
| Polyvinyl chloride plastic compound | 3,0-5,0 | |
| Polyvinyl chloride plastic compound over a polyisobutylene sublayer | 10 | |
| Lining1) | Ceramic tiles (acid-resistant or for floors) on binders2) | 20-60 |
| Acid-resistant brick with binders2) | — | |
| Piece acid-resistant ceramic materials, straight tiles, shaped tiles, acid-resistant bricks3) on a chemically resistant binder on an underlayer (non-vulcanized chemically resistant rubber based on polyisobutylene, bitumen roll insulation, etc.) | 30-270 | |
| Slag-sitall tiles with epoxy binders over an underlayer of paint and varnish composition reinforced with fiberglass | 12-20 | |
| Acid-resistant tiles made of stone casting with silicate putty on the sublayer (non-vulcanized chemical-resistant rubber based on polyisobutylene, etc.) | 30 | |
| Carbon-graphite materials (ATM tiles, carbon and graphite blocks) on putties based on polymer materials on the sublayer (polyisobutylene, etc.) | 20-400 | |
| Gumming | Rubbers and ebonites on adhesives with subsequent vulcanization | 3-12 |
| 1) The protective coating scheme, thickness and number of layers should be selected taking into account the dimensions of the structure, temperature, characteristics of the aggressive environment with mandatory checking for static stability, and, if necessary, with thermal calculations. 2) The binder should be selected taking into account the composition of the aggressive environment. 3) Piece acid-resistant materials should be selected depending on the nature of the media, mechanical loads and thermal calculations. | ||
Table T.10 - Methods of corrosion protection of load-bearing and enclosing structures made of thin-sheet cold-rolled steel
| Operating conditions index by | Constructions | |
| table X.9 | carriers | fencing1) |
| C1 (in the absence of condensation) | Hot zinc coatings with a thickness of at least 24 microns or a class of at least 350; hot zinc coatings with a thickness of at least 19 microns (or class of at least 275) with additional paint coating of groups II and III according to table Ts.8; hot-dip galvanized coatings with a thickness of at least 19 microns (or class of at least 275) with additional paint coating of groups II and III according to Table C.1 | Hot zinc coatings with a thickness of at least 19 microns or class of at least 275; hot aluminum-zinc coatings from a melt containing 55% aluminum, 43.4% zinc and 1.6% silicon, with a thickness of at least 25 microns or a class of at least 185; hot zinc coatings with a thickness of at least 7 microns or class of at least 100 with additional paint coating of groups II and III according to table Ts.8; electrolytic zinc coatings with a thickness of at least 7 microns with additional paint coating of groups II and III according to table C.8 |
| C2 | Hot-dip galvanized coatings with a thickness of at least 19 microns (or class of at least 275) with an additional paint coating of groups II and III according to table Ts.82); hot zinc coatings with a thickness of at least 19 microns (or class of at least 275) with an additional paint coating of groups II and III according to Table C.1 with a thickness of at least 80 microns | Hot zinc coatings with a thickness of at least 19 microns (or class of at least 275) with an additional paint coating of groups II and III according to Table Ts.8; hot zinc coatings with a thickness of at least 19 microns (or class of at least 275) with an additional paint coating of groups II and III according to Table C.1 with a thickness of at least 60 microns |
| C3 | Hot zinc coatings with a thickness of at least 24 microns (or class of at least 350) with additional paint coating of groups III, IV according to Table Ts.8; hot zinc coatings with a thickness of at least 24 microns (or class of at least 350) with an additional paint coating of groups III, IV according to Table C.1 with a thickness of at least 120 microns | Hot-dip galvanized coatings with a thickness of at least 19 microns (or class of at least 275) with additional paint coating of groups II, III, IV according to Table Ts.8; hot-dip galvanized coatings with a thickness of at least 19 microns (or class of at least 275) with an additional paint coating of groups II, III, IV according to Table C.1 with a thickness of at least 100 microns |
| C4 | Not allowed for use | Not allowed for use |
| C5 | Not allowed for use | Not allowed for use |
| 1) In accordance with the requirements of table X.8. 2) The thickness of the paint and varnish coating is the same as for operating conditions with the SZ index. Notes 1 The group and thickness of the paint coating are given in Table Ts.8. 2 For environments with a non-aggressive degree of impact, the thickness of the paint coating layer should be established in accordance with the relevant regulatory documents. | ||
Table C.11 - Reference data on the rate of corrosion penetration of carbon steel and zinc coatings under various operating conditions
| Conditions index | Maximum corrosion penetration rate, microns per year | |||||
| operation according to table X.9 | Carbon steel | Hot Dip Galvanized | Galvanic (electrolytic) zinc coating | Thermal diffusion zinc coating | Thermal diffusion zinc coating with additional coating with zinc-containing primer | |
| Name of galvanized products | ||||||
| Thin sheets1) | Profiles and fasteners | Thin sheet metal2) and fasteners | Profiles and fasteners | Fasteners | ||
| C1 | 10 | 0,4 | 0,4 | 1,0 | 0,3 | 0,3 |
| C2 > | 25 | 1,0 | 0,8 | 1,5 | 0,6 | — |
| C3 | 50 | 3,3 | 2,5 | 5 | 1,7 | — |
| C4 | 500 | 35 | 25 | 50 | 18 | — |
| C5 | Over 500 | St. 35 | St. 25 | Over 50 | St. 18 | — |
| 1) Thin sheets are galvanized on continuous hot-dip galvanizing lines for rolled products. 2) Thin-sheet rolled products are galvanized on continuous lines for electrolytically galvanizing rolled steel. | ||||||
Tables Ts.10, Ts.11 (Introduced additionally, Amendment No. 1).
<<back / to contents SP 28.13330.2017 / forward >>
Table 2. Compatibility of steel with metals
| Metals for which data are presented in the table on their susceptibility to corrosion | Ratio of metal area to other metals table | Carbon steel | Low alloy steel | Cast steel | Chrome steel | Stainless steel | |||||||
| Magnesium | Low | WITH | WITH | WITH | WITH | WITH | |||||||
| High | WITH | WITH | WITH | WITH | WITH | ||||||||
| Zinc | Low | WITH | WITH | WITH | WITH | WITH | |||||||
| High | N | N | N | N | N | ||||||||
| Aluminum | Low | U | WITH | WITH | |||||||||
| High | N | N | U | U | U | ||||||||
| Cadmium | Low | WITH | WITH | WITH | WITH | WITH | |||||||
| High | N | N | N | N | N | ||||||||
| Carbon steel | Low | U | WITH | WITH | WITH | ||||||||
| High | N | N | N | N | |||||||||
| Low alloy steel | Low | N | N | WITH | WITH | ||||||||
| High | N | N | N | N | |||||||||
| Cast steel | Low | N | U | WITH | WITH | ||||||||
| High | N | N | N | ||||||||||
| Chrome steel | Low | N | N | N | WITH | ||||||||
| High | N | N | N | N | |||||||||
| Lead | Low | N | N | N | N | ||||||||
| High | N | N | U | N | N | ||||||||
| Tin | Low | N | N | N | |||||||||
| High | N | N | N | U | |||||||||
| Copper | Low | N | N | U | |||||||||
| High | N | N | N | N | |||||||||
| Stainless steel | Low | N | N | ||||||||||
| High | N | N | N | U | |||||||||
| Column 1 of the table presents metals that are or are not subject to corrosion with the metals indicated in the remaining columns of the table and the proportion of the ratio of the areas of the metal indicated in column 1 to the metals in the remaining columns of the table. The short designation S, U, N in the table means:
| |||||||||||||
Methods of applying anti-corrosion protection to metal structures
Application by brush
The most popular application method is to use a synthetic fiber brush.
Roller application
Use any fine-pile construction roller
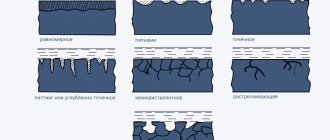
Types of metal corrosion
Complete corrosion
The least dangerous thing for various metal objects is complete corrosion. It is especially not dangerous for those situations where damage to devices and equipment does not violate the technical standards for their further use. The consequences of this type of corrosion can be easily predicted and equipment adjusted accordingly.
Local corrosion
The greatest danger is local corrosion. In this case, the loss of metal is not large, but through damage to the metal is formed, which leads to failure of the product or equipment. This type of corrosion occurs in products that come into contact with sea water or salts. This appearance of rust causes the surface of the metal base to partially corrode and the structure loses its reliability.
A large number of problems arise in places where sodium chloride is used. This substance is used to remove snow and ice on roads in urban areas. This type of salt causes them to turn into liquid, which, already diluted with salts, ends up in city pipelines. In this case, protecting metals from corrosion will not hurt. All underground communications begin to collapse when water and salts enter. In the United States of America, it is estimated that approximately two billion dollars are spent annually on road repairs. However, utility companies are not yet ready to abandon this type of salt for treating road surfaces due to its low cost.
Types and causes of corrosion on metal products
When taking measures to protect metal products from corrosion, you need to know what exactly it is. The following types of corrosion exist:
- Liquid. Forms on metal surfaces in contact with a damp environment. As for seawater, the oxidation process occurs much faster in it due to the increased concentration of salt in the liquid.
- Soil. This type is typical for metal structures that interact with the ground for a long time. When exposed to chemical elements contained in groundwater, soil or various leaks, irreversible chemical processes are triggered.
- Atmospheric. The main cause of oxidation is the interaction of the metal with water vapor and oxygen contained in the air. If the air is saturated with contaminants of chemically active substances, then rust appears faster.
The manifestation of corrosion on metal structures can be expressed as:
- the formation of a continuous layer of rust or individual areas of surfaces;
- the appearance of deep cracks;
- small affected areas directed towards the inside of the product;
- oxidation of one of the alloy components;
- deep distribution throughout the entire volume;
- several signs at once.
One of two reasons for the development of such a process may be:
- Chemical interaction - when a metal begins to deteriorate due to a chemical reaction with active components.
- Electrochemical nature, due to the fact that upon contact with electrolytic solutions, electric currents are generated, under the influence of which electrons are replaced in the metal. This leads to destruction of the crystal lattice and the formation of rust.
Methods for protecting metals from corrosion
Since ancient times, people have tried to protect metals from corrosion. Constant precipitation rendered metal products unusable. That is why people lubricated them with various fatty oils. Then they began to use coatings of other metals that do not rust for this purpose.
Modern chemists carefully study all possible methods of combating metal corrosion. They create special solutions. Methods are being developed to reduce the risk of corrosion on metals. An example would be a material such as stainless steel. For its production, iron was used, supplemented with cobalt, nickel, chromium and other elements. With the help of elements added to it, it was possible to create a metal on which rust does not form for a longer period of time.
To protect various metals from corrosion, various substances have been developed that are actively used in modern industry. Varnishes and paints are actively used today. They are the most affordable means of protecting metal products from rust. They create a barrier for water or air to reach the metal itself. This allows you to temporarily delay the appearance of corrosion. When applying paint or varnish, you should take into account the thickness of the layer and the surface of the material. To achieve the best result, the coating of metals against corrosion should be done in an even and dense layer.
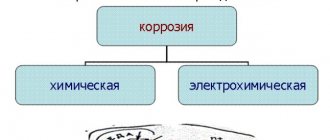
Definition of corrosion
Corrosion is the gradual destruction of objects, usually metals, caused by an active electrolyte environment and a chemical oxidation reaction.
The essence of the corrosion process is the presence of a constantly operating anodic reaction. It is caused by the dissolution of a metal, which generates electrons. Part of the activation energy is additionally spent on another process called the cathodic reaction. These two processes balance the charges produced. The zones causing these processes can be located close or far from each other, depending on the situation.
The electrons generated in the process must be consumed through a cathodic reaction. Hydrogen ions and electrons react to form atomic and then hydrogen gas. However, hydrogen is a powerful reducing agent, so further corrosion can be prevented by creating a thin film of gas on the metal surface. It serves as a polarizer, reducing metal contact with water and reducing corrosion. Thus, anything that destroys the barrier film increases the rate of corrosion.
The main factors determining the intensity of the process are:
- Speed;
- Temperature;
- The level of mechanical and thermal stresses that arise;
- The nature of the ongoing chemical reactions.
Corrosion hinders the introduction of new metal materials into production and causes significant damage to the economy.
Chemical corrosion of metals
Essentially, corrosion can be of two types:
- chemical,
- electrochemical.
Chemical corrosion is the formation of rust under certain conditions. In industrial environments, it is not uncommon to encounter this type of corrosion. Indeed, in numerous modern enterprises, metals are heated before creating products from them, which leads to the formation of such a process as accelerated chemical corrosion of the metal. This produces scale, which is a product of its reaction to the appearance of rust during heating.
Scientists have proven that modern iron is much more susceptible to rust. It contains a large amount of sulfur. It appears in the metal due to the fact that coal is used during the extraction of iron ores. The sulfur from it gets into the iron. Modern people are surprised that ancient objects made from this metal, which archaeologists find at excavations, retain their external qualities. This is due to the fact that in ancient times charcoal was used to extract iron, which contains virtually no sulfur that could get into the metal.
Anti-corrosion paint as protection for metal structures
Anti-corrosion paint is a liquid material applied to metal using traditional paint methods, protecting it from corrosion, that is, it is a corrosion inhibitor. In the vast majority of cases, anti-corrosion paint is given the desired color by adding color pigments. The AKTERM company recommends using 3 in 1 enamel primer AKTERM Plast as an anti-corrosion protection for metal structures
AKTERM Primer-enamel Plast
A one-component, quick-drying decorative coating, used as anti-corrosion protection for metal structures, bridges, cell phone towers, ship hulls, vehicle bodies and rolling stock operating under the influence of external climatic factors. Weather resistance up to 10 years. Colored in RAL. Universal anti-corrosion protection of metal
Read more
Anti-corrosion paints can have heat-reflecting (heat-insulating) properties, in addition to anti-corrosion - the AKTERM Anticor material has such properties
AKTERM Anticor
Heat-reflecting coating to protect metal surfaces from corrosion, operating temperature from -50ºС to +150ºС Thermal insulation + anti-corrosion properties of metal
Read more
Anti-corrosion paint can also have electro-chemical protective properties, in which case zinc is applied (cold galvanizing) as a metal coating - in fact, zinc paint is used, which is called a cold galvanizing composition.
AKTERM Zinc
The composition of cold galvanizing, which is based on 96% zinc, has electrochemical protection of the metal, as well as a protective effect - comparable in protective properties to hot and galvanic methods of galvanizing. The composition is suitable for outdoor and indoor ventilated areas. Electro-chemical protection of metal + anti-corrosion of metal
Read more
Polyurethane compounds are also used to give the anti-corrosion material increased abrasion resistance properties and prevent metal destruction - AKTERM Anticor PU - this composition can be classified as a “metal corrosion protector”.
AKTERM Anticor PU
Two-component coating, used as an independent protective and decorative anti-corrosion protector for external surfaces, tanks, tanks, cars, bodies and components of vehicles and rolling stock, structures made of steel, cast iron, aluminum and titanium alloys, operated in all types of atmosphere and load categories C2 -C4. Protection up to 20 years. Colored in RAL. Abrasion resistance + fracture prevention + anti-corrosion
Read more
These metals are susceptible to corrosion.
There are various types of metals. Most often, iron is used to create any items or objects. It is from it that twenty times more products and objects are made than from other metals combined. This metal began to be used most actively in industry in the late 18th and early 19th centuries. It was during this period that the first cast iron bridge was built. The first sea vessel appeared, for the manufacture of which steel was used.
In nature, iron nuggets are found in rare cases. Many people believe that this metal is not terrestrial, it is classified as cosmic or meteorite. It is this that is most susceptible to corrosion.
There are also other metals that are susceptible to corrosion. Among them, copper, silver, and bronze stand out.
Paints for coating metal products
Paints intended for treating metal surfaces are standard and heat-resistant. In most cases, three types of compounds are used: epoxy, acrylic and alkyd. There are also special anti-corrosion paints that have the following advantages:
- effectively protect the coating from atmospheric influences and temperature changes;
- can be easily applied with a roller, brush or spray;
- many of them are quick-drying;
- have a wide selection of colors;
- are durable.
As for the most inexpensive and accessible means, then you should pay attention to ordinary silver. This coating contains aluminum powder, which forms a protective film on the product treated with it.