Aluminum is a material that people often use in industry and for their own needs. This metal is flexible and resistant to external influences. It is non-toxic and safe for human health. Silver color allows the metal to be used for various purposes. These are industry and household spheres.
When working in industry, people often wonder if aluminum rusts. Everyone knows that if damage appears on a sheet, corrosion can develop. You should learn why aluminum rusts differently than other alloys. It is necessary to find out the reasons why it is subject to corrosion. Read about all this and more in our article today.
Properties
Let's study the characteristics of aluminum. The described metal melts at a temperature of 659 degrees Celsius. The density of the substance is 2.69*103 kg/cm3. Aluminum belongs to the group of active metals. Resistance to corrosion processes depends on a number of factors:
- Purity of the alloy. For the production of various equipment, metal is taken that is distinguished by its purity. It should not contain various impurities. Aluminum grades AI1 and AB2 are widely used.
- Environment in which aluminum is located.
- What is the concentration of impurities in the environment surrounding aluminum.
- Temperature.
- The pH of the environment has a great influence. You need to know that aluminum oxide can form when the pH is in the range between 3 and 9. In an environment where an oxide film immediately appears on the surface of an aluminum sheet, corrosion processes will not develop.
Organic compounds
Non-corrosive:
- All saturated hydrocarbons, benzene, toluene, naphthalene.
- Aliphatic alcohols (interaction with aluminum is possible only after evaporation of water and only when heated). Methyl alcohol causes minor corrosion in aqueous solution, most severely at 25% water content. Other alcohols, such as ethyl, propyl, glycerin and its derivatives, do not corrode aluminum.
- Phenol and its aqueous solutions (minor destruction occurs at temperatures above 60°C and a concentration of 50%). Phenol derivatives behave similarly.
- Esters.
- Aldehydes, including formalin, acrolein, benzaldehyde.
- Ketones.
- Freons.
- Amines and amides.
- Certain halogen-containing compounds (eg polyvinyl chloride).
- Carbohydrates: glucose, lactose, cellulose.
Lead to minor corrosion:
- Some organic chlorine compounds that release hydrochloric acid due to hydrolysis in aqueous solutions.
- Aqueous solutions of organic acids: acetic, formic, monochloroacetic, oxalic, tartaric, citric, malic, salicylic, ascorbic, etc.
Strong effect on aluminum:
- Some organic acids in the complete absence of water.
- Salts of organic acids can create sources of local corrosion.
- Carbon tetrachloride is highly corrosive in the presence of traces of moisture.
- Most halogen compounds attack aluminum, and the corrosion rate increases with temperature. The corrosive activity of halogen decreases in the following sequence: F-Cl-Br-I.
- Some sulfur-containing organic compounds, such as ethyl sulfuric acid.
How is aluminum protected from corrosion?
Alloys of other metals are susceptible to rust. It manifests itself quite quickly. If you create certain conditions for aluminum, it will not deteriorate for many years. To protect aluminum from corrosion, a special film is formed on it. It lays down in a thin layer, which ranges from 3 to 30 nanometers. This coating consists of aluminum oxide.
The film is durable and gives the metal additional protection from external negative influences. Thanks to this layer, air and moisture do not enter the structure of the material. If the integrity of the oxide coating is compromised, then the process of aluminum corrosion begins. The metal loses its properties.
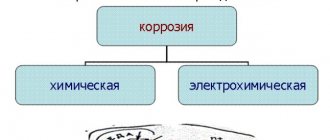
Three main types of aluminum corrosion
The most common types of aluminum corrosion are:
- galvanic (contact) corrosion;
- pitting (pitting) corrosion;
- crevice corrosion.
Stress corrosion, which leads to cracking, is a more specific type of corrosion. It occurs primarily in high-strength aluminum alloys, such as AlZnMg alloys, when they are subjected to prolonged tensile stress in the presence of a corrosive environment. This type of corrosion does not usually occur in 6xxx series alloys, i.e. AlMgSi alloys.
Causes of corrosion
When the question arises whether aluminum rusts, you need to think about the reasons leading to corrosion. Various external factors can accelerate this process. The reasons for the appearance of rust on aluminum can be the following:
- Interaction with any acid or alkali.
- Mechanical pressure. For example, friction or a strong impact, after which a scratch appears on the top layer of metal.
- There are industrial areas. In them, fuel breakdown products affect the oxide film and destroy it. The metal begins to deteriorate. A similar situation occurs in megacities, where fuel breakdown products will interact with sulfur, as well as carbon oxides. A similar process destroys the film on aluminum. After this kind of external exposure, aluminum undergoes corrosion.
- It should be remembered that chlorine, fluorine, as well as bromine and sodium can dissolve the protective layer of the metal.
- If construction mixtures come into contact with metal, it quickly begins to deteriorate. In this case, aluminum is adversely affected by cement.
- Does water rust aluminum? If it gets on the sheet, the metal may be subject to corrosion processes. It is important to clarify which liquid has an effect. Many people use a special alloy that is not susceptible to corrosion from water. It is called duralumin. A unique alloy is used together with copper, as well as manganese.
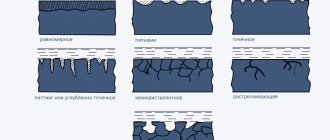
Pit corrosion of aluminum
For aluminum, pitting corrosion is the most common type of corrosion. It also occurs only in the presence of an electrolyte (water or moisture) that contains dissolved salts, usually chlorides.
This corrosion usually appears as very small pits that, when exposed to air, reach a maximum depth of a small fraction of the thickness of the metal. The depth of these pits can be greater in water and soil.
Prevention of pitting corrosion
Pitting corrosion is mainly an aesthetic issue because, in a practical sense, it never reduces the strength of aluminum products.
The manifestation of pitting corrosion, of course, is more serious on aluminum with a natural surface, that is, a surface without any protective treatment. Protective surface treatment of aluminum (anodizing, painting or other coating methods) successfully protects it from pitting corrosion.
To prevent pitting corrosion, cathodic protection is also used (see above).
Drainage design
It is very important to design aluminum profiles and other aluminum products so that they have the ability to drain precipitation and quickly dry the surface. Profiles that may be exposed to moisture should not have corners or pockets in which water accumulates. Each profile in which water can accumulate must have drainage holes (Figure 3).
Figure 3 – Structural drainage in aluminum profiles
Effective drainage (Figure 4) and ventilation of “wet” aluminum profiles significantly reduces the risk of pitting corrosion on them.
Figure 4 – Drainage holes in aluminum profile
What is electrochemical corrosion and can it occur on an aluminum sheet?
Most often, the appearance of electrochemical corrosion is provoked by galvanic couples. Damage occurs at the junction of two different alloys. In this case, the rust will be clearly visible. The important point is that only one metal deteriorates, and the second is the source of the corrosion process. In order not to be afraid of electrochemical corrosion, you need to use a magnesium alloy. Due to electrochemical rust, experts do not recommend using ordinary iron when in contact with an aluminum body.
Electrochemical corrosion
Technical aluminum (or its alloys) contains metal impurities in the form of individual inclusions (magnesium, titanium, iron, manganese, etc.)
Due to the presence of such inclusions, the alloy immersed in the electrolyte is a collection of a large number of microscopic galvanic foci. As a result of the electrochemical reaction that occurs in these centers, the metal acting as the anode (and in our case it is the main component of the alloy, aluminum) dissolves, while hydrogen is released at the microcathodes ( Fig. 2 ).
Such micro-sources of corrosion are, by their nature, ordinary galvanic elements and differ in:
- 1 microscopic dimensions of the anode and cathode;
- 2 horizontal arrangement of electrodes;
- 3 direct connection of cathode and anode.
The process of electrochemical corrosion is not always the result of the appearance of microscopic galvanic elements. In some cases, corrosion foci have “visible” (macroscopic) dimensions.
The mechanism of electromechanical corrosion destruction for different surface sizes of the cathode (steel) and anode (aluminum) is shown in Fig. 3 .
What factors can slow down the process?
There are a number of factors that slow down the corrosion process of aluminum, and some of them stop this phenomenon. The following are distinguished:
- In order for the corrosion-inhibiting properties of aluminum to be maintained, it is necessary to maintain the acid-base balance. The range should be between six and eight units.
- It is believed that pure metal, without impurities, better withstands aggressive environments. Scientists conducted experiments. Based on the results, we can say that alloys of pure aluminum (90%) are more susceptible to corrosion than an alloy containing 99% of this substance. In the first option, corrosion occurs 80 times faster than in the second alloy.
- To ensure that the metal does not lose its properties longer in an aggressive environment, it is treated with a special paint. You can use a polymer composition. After processing, an additional protective layer appears.
- If you add 3% manganese to the alloy during production, it will be possible to avoid corrosion of aluminum.
Synthetic chemicals and industrial products
Does not affect aluminum:
- Synthetic resins.
- Insecticides (except sodium arsenate).
- Cosmetic preparations.
- Lucky.
- Organic dyes.
- Some adhesives that do not contain heavy metal salts.
Affect aluminum moderately or strongly:
- Cleaners.
- Detergents.
- Some cosmetic preparations (acidic or alkaline).
- Cologne.
- Inorganic dyes.
- Ink and ink.
- Adhesives.
- Products of combustion of organic substances, especially sulfur- and nitrogen-containing substances.
Under what conditions does aluminum deteriorate in air?
Some people wonder if aluminum rusts when exposed to air. If the oxide film on the top layer of metal is destroyed, the corrosion process may begin. As a result, rust may appear. Film growth tends to slow down in fresh air. It should be remembered that aluminum oxide has good adhesion to the metal surface.
If the sheet is stored in a warehouse, the film will be from 0.01 to 0.02 microns. If the metal comes into contact with dry oxygen, then the thickness of the oxide film on the surface will be from 0.02 to 0.04 microns. If aluminum is subjected to heat treatment, the film thickness changes. It will be equal to 0.1 microns.
Aluminum is considered to be durable enough to be used outdoors. For example, it is used in rural areas, as well as in remote industrial areas.
Natural materials
Does not work on aluminum:
- Rock salt.
- Petroleum distillation products: gasoline, kerosene, paraffin, oils, resins.
- Wax.
- Rubber.
- Essential oils.
- Hard coal, anthracite, brown coal.
- Cellulose.
- Squirrels.
- Natural gypsum.
- Particularly pure types of oil.
Effective on aluminum:
- Oil, or more precisely, the contaminants it contains, mainly water with salts dissolved in it, which form acids as a result of hydrolysis.
- Tannins.
- Wood impregnation products.
- Clay.
How does water affect the metal being described?
Corrosion of aluminum in water can occur from damage to the top layer and protective film. The high temperature of the liquid contributes to the rapid destruction of the metal. If aluminum is placed in fresh water, then corrosion processes will practically not be observed. If you increase the water temperature, you may not notice any changes. When the liquid heats up to a temperature of 80 degrees or higher, the metal begins to deteriorate.
The corrosion rate of aluminum increases if alkali gets into the water. The described metal is hypersensitive to salt. That is why sea water is destructive for it. To use this metal in seawater, it is necessary to add magnesium or silicon to the liquid. If you use a sheet of aluminum that contains copper, then corrosion of the alloy will occur much faster than that of a pure substance.
Crevice corrosion of aluminum
The essence of crevice corrosion
Crevice corrosion can occur in narrow, fluid-filled crevices. The occurrence of such corrosion in aluminum profiles is unlikely. However, significant crevice corrosion can occur in the marine atmosphere or on the exterior surfaces of vehicle bodies. During transportation and storage of aluminum profiles, water can sometimes collect in the crevices between adjacent aluminum surfaces, causing surface corrosion in the form of “water spots” (Figure 4).
Figure 5 – Essence of crevice corrosion
The source of this water is rain or condensation. This water is literally sucked through a capillary mechanism into the space between two metal surfaces. Moisture condensation can occur when cold material is placed in a warm room. The difference between night and day temperatures can also cause condensation when aluminum is stored outside under a thick awning that prevents ventilation.
Is sulfuric acid dangerous for aluminum?
People wonder whether aluminum rusts in sulfuric acid. Such acid is potentially dangerous for alloys. It has pronounced oxidizing properties. They destroy the oxide film and accelerate metal corrosion.
An interesting point is that concentrated cold sulfur does not affect aluminum. If aluminum is heated, then metal corrosion processes can begin. In this case, salt appears, it is called aluminum sulfate. It is soluble in water.
Chemical resistance of aluminum alloys
Water
In distilled water, aluminum exhibits very good corrosion resistance at any temperature.
Rainwater can destroy aluminum if the atmosphere contains significant amounts of industrial gases. Dissolving in water, these gases (SO2, NO2, hydrogen chloride, etc.) form acids that destroy aluminum. Therefore, to avoid corrosion, aluminum structures should be designed to minimize the accumulation of rainwater on the metal surface.
Tap water affects aluminum differently, depending on the impurities it contains. Aluminum can corrode in acidic or alkaline waters. The corrosion process is accelerated by chlorine ions or heavy metals contained in tap water.
Industrial wastewater causes very strong corrosion, which is accelerated by heavy metal ions.
Gases
Hydrogen, nitrogen and noble gases (helium, argon, neon, krypton, xenon) do not affect aluminum even at elevated temperatures.
Halogens (chlorine, bromine, iodine, fluorine) have no effect on aluminum in the absence of moisture. When interacting with water, they form acids that are aggressive towards aluminum.
Dry hydrogen chloride, hydrogen bromide, hydrogen iodide, and hydrogen fluoride have no effect on aluminum. But aqueous solutions of these gases are acids that actively destroy aluminum.
Hydrogen sulfide does not destroy aluminum at temperatures up to 500°C.
Sulfur dioxide does not destroy aluminum in the absence of water vapor (up to 400°C), although it causes corrosion in the presence of moisture. Sulfur trioxide acts similarly.
Ammonia in the gaseous state has no effect on aluminum even at high temperatures.
Carbon monoxide CO destroys aluminum only at temperatures above 550°C.
Carbon dioxide behaves similarly to CO. In water, carbon dioxide forms carbonic acid, which does not cause significant corrosion damage.
Will metal corrosion occur upon contact with alkali?
Do not allow aluminum to come into contact with various alkalis. They easily destroy the protective film on the top layer. The metal reacts with water, after which hydrogen begins to be released. The corrosion process occurs quickly in this case. Mercury and copper also have a detrimental effect on the protective layer of aluminum.
So, we found out whether aluminum rusts. As you can see, it does not always have good corrosion protection.
Methods for protecting materials from corrosion
Aluminum is a type of alloy that can withstand the risk of corrosion under different conditions - in the soil, in the open air, in contact with water.
Three recommendations to help significantly increase the degree of protection of the material:
- Taking into account the characteristics of the alloy and the area of use . Depending on the type of environment, different elements in the alloy can act as additional corrosion catalysts. Thus, increased copper content may increase the risk of problems when in contact with seawater.
- Elimination of unfavorable neighborhoods . In particular, you should not use products nearby whose materials can create cathode-anode bonds with aluminum.
- Application of special coatings . They prevent contact between the base alloy and factors that cause rust. Various mastics, powder, and anodic oxide coatings are used. It is also important to take into account the conditions of their application and properly prepare the surface to increase the degree of adhesion.
If the problem does arise, it will be possible to solve the question of how to remove corrosion from aluminum.
For this purpose, mechanical cleaning and special inhibitor compounds are used, which can significantly increase the degree of protection and prevent further spread of damage.
Return to articles Share article