Polymers are high molecular weight chemical compounds (HMCs), the macromolecules of which are formed from many monomer units. Polymer molecules are characterized by a huge molecular weight, from several thousand to several million atomic mass units . There are several options for classifying polymers.
- Based on their chemical composition, polymers are divided into organic ( polyethylene ), inorganic (silicates) and organoelement ( fluoroplastic-4) .
- Depending on their origin, polymers are natural, artificial (modified) and synthetic.
- The classification of polymers according to the composition of their monomer units divides polymers into homopolymers and heteropolymers (or copolymers).
- Depending on the structure of the main chain, homochain and heterochain polymers are distinguished.
- Based on the spatial structure of monomer units , polymers are divided into stereoregular and nonstereoregular (or atactic).
- According to the structure of macromolecules, polymers are divided into: linear, branched, ladder and three-dimensional cross-linked (mesh, spatial).
- Depending on the production reaction, polymers are also divided into polymerization and polycondensation.
- The classification of polymers in relation to temperature effects is of important practical importance. In relation to heating allocate thermoplastic (polyethylene, polyvinyl chloride, polystyrene) And thermosetting polymers (epoxy resins).
What are polymers
Synthetic polymers are petroleum derivatives. Most of them are obtained through two main reactions - polymerization and polycondensation.
Substances that have multiple monomer bonds enter into the polymerization reaction. As a result, one product is obtained.
The polycondensation reaction involves substances that have functional groups of monomers in the chain. In this case, the output is a high molecular weight polymer and a low molecular weight substance (water).
Polymers are not only artificially created substances by man, but also natural building materials for all living things.
These include:
- complex carbohydrates
– polymers of elemental sugars;
- squirrels
– polymers of amino acids;
- cellulose
– polymer found in wood;
- keratin
– is a polymer and is found in the hair, nails and feathers of birds;
- chitin
– a polymer that holds together the shells of arthropods;
- nucleic acid derivatives
– DNA heteropolymers.
Discovery of polymers
The 20th century can rightfully be called the century of polymers. Their discovery was not a deliberate study. They were originally a byproduct of various experiments and chemical reactions.
Chemist Leo Baekeland eventually turned his attention to these useless materials, and his experimental work resulted in plastic, a polymer that can take on different shapes when temperature and pressure change. With the invention of Bakelite (the original name of plastic), the era of polymer production began.
During World War II, synthetic rubber was developed for the production of rubber to meet the needs of the American army. During unsuccessful experiments, a new polymer was also discovered in the form of a mastic with increased elasticity. This was the time of the creation of hardboard glass and phenol-formaldehyde-based resins. A separate branch has emerged in chemistry – polymers.
Classification of polymers
Polymers have several classifications.
First of all, they are divided by origin:
- natural
– rubber, proteins, potato and corn starch, wood cellulose;
- artificial
– viscose;
- synthetic
- nylon, polyurethanes.
Next by molecular weight. It indicates how homogeneous a molecule is in its chemical composition. The percentage of its reaction and polymerization depends on the number of repetitions of one structural unit of monomers in their construction. Therefore, additional properties play an important role in classification.
Polymers in various branches of science and technology
We have already touched upon the question of in what areas polymers are used. Examples showing their importance in science and technology include the following:
- use of rubber;
- antistatic coatings;
- electromagnetic screens;
- housings of almost all household appliances;
- transistors;
- LEDs and so on.
There are no limits to imagination regarding the use of polymer materials in the modern world.
Reaction to heat treatment
There are two types:
- Thermoplastic
– after heating they return to their original shape. Can be recycled many times.
- Thermoset
– They are destroyed under the influence of high temperatures.
As a result of various processing processes, the resulting polymers are divided into 4 main groups:
- plastics
– polypropylene, polystyrene, polyvinyl chloride, polyurethane;
- fibers
– acetate silk, viscose;
- elastomers
– rubber, rubber (vulcanized rubber);
- biopolymers
- carbohydrates, proteins, nucleic acids.
Examples of products made from polymer materials
Before naming specific products made from polymers (it is impossible to list them all, there is too much variety), first you need to understand what the polymer provides. The material that is obtained from the Navy will be the basis for future products.
The main materials made from polymers are:
- plastics;
- polypropylenes;
- polyurethanes;
- polystyrenes;
- polyacrylates;
- phenol-formaldehyde resins;
- epoxy resins;
- nylons;
- viscose;
- nylons;
- polyester fibers;
- adhesives;
- films;
- tannins and others.
This is just a small list of the diversity that modern chemistry offers. Well, here it already becomes clear what objects and products are made from polymers - almost any household items, medicine and other areas (plastic windows, pipes, dishes, tools, furniture, toys, films, etc.).
Properties of polymers
Depending on whether polymers have an organic composition or are derivatives of inorganic compounds, their basic properties appear:
- have high strength under mechanical stress;
- there is no precisely defined melting point;
- the main part is insoluble in water;
- retain the ability to viscous flows;
- do not change their qualities after heating and cooling;
- dielectrics;
- plastic, easy to form;
- waterproof.
They can be soft, hard, flexible, rigid or strong.
Physical properties of polymer materials
The main two states of aggregation characteristic of polymers are:
- amorphous;
- crystalline.
Each is characterized by its own set of properties and has important practical significance. For example, if a polymer exists in an amorphous state, it means that it can be a viscous flowing liquid, a glass-like substance, or a highly elastic compound (rubbers). It is widely used in the chemical industries, construction, engineering, and the production of industrial goods.
The crystalline state of polymers is rather conditional. In fact, this state alternates with amorphous sections of the chain, and in general the entire molecule turns out to be very convenient for producing elastic, but at the same time high-strength and hard fibers.
Melting points for polymers are different. Many amorphous ones melt at room temperature, and some synthetic crystalline ones can withstand fairly high temperatures (plexiglass, fiberglass, polyurethane, polypropylene).
Polymers can be painted in a variety of colors, without restrictions. Thanks to their structure, they are able to absorb paint and acquire the brightest and most unusual shades.
Application of polymers
Without these compounds, modern civilization cannot develop and exist. Products based on raw materials with various monomer compounds are necessary both in everyday life and for the operation of high-tech industries.
The proposed table only to a small extent reflects examples of their application.
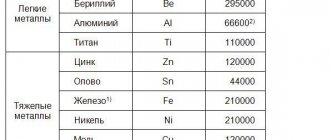
| Name of polymer compounds | Scope of application |
| Polyethylene | Packaging materials, insulation of electrical wires, machine parts, containers for storing acids and alkalis, corrosion protection of oil pipelines. |
| Polystyrene | Toys, parts of household appliances, interior lining of cars and airplanes, fittings, electronics cases, dishes. |
| Polyvinyl chloride | Machine parts, equipment for the chemical industry, artificial leather, PVC window frames. |
| Polymethyl methacrylate | Plexiglas, lighting components, cladding of aircraft and cars. |
| Polycarbonates | Particularly precise parts of machines and electronics, replacement of metal structures, building materials. |
| Epoxy resins | Varnishes, glue, laminate. |
| Polyesters | Lamps, masts, fishing rods, protective equipment, aircraft and machine bodies. |
X and M and I
Organic chemistry
Polymers.
Polymers
(Greek πολύ- - many; μέρος - part) are complex substances whose molecules are built from many repeating elementary units -
monomers
.
Polymers
are high molecular weight compounds with large molecular weights (of the order of hundreds, thousands and millions).
The following two processes lead to the formation of high molecular weight compounds:
1. Polymerization reaction,
2. Polycondensation reaction.
Polymerization reaction
Polymerization reaction
– a process as a result of which molecules of a low molecular weight compound (
monomer
) combine with each other, forming a new substance (
polymer
), the molecular weight of which is an integer number of times greater than that of the monomer.
Polymerization
, is mainly characteristic of compounds with multiple bonds (double or triple). During the polymerization reaction, multiple bonds are converted into simple (single) bonds. The valence electrons released as a result of this transformation are used to establish covalent bonds between monomers.
An example of a polymerization reaction is the formation of polyethylene from ethylene:
Or in general:
A characteristic feature of this reaction is that as a result only the polymer substance is formed and no by-products are released.
. This explains the multiple weights of the polymer and the original monomers.
Polycondensation reaction
Polycondensation reaction
– the process of polymer formation from low molecular weight compounds (monomers).
But in this case, monomers contain two or more functional groups, which during the reaction lose their atoms, from which other substances are formed (water, ammonia, hydrogen halides, etc.).
Thus, the composition of the elementary unit of the polymer differs from the composition of the original monomer, and during the polycondensation reaction we obtain not only the polymer itself, but also other substances
.
An example of a polycondensation reaction - the formation of nylon
from
aminocaproic acid
:
During this reaction, the amino group ( -NH2
) loses one hydrogen atom, and the carboxyl group (
-COOH
) loses its hydroxyl group (
-OH
). The ions separated from the monomers form a water molecule.
Natural polymers
Examples of natural high-molecular compounds (polymers) are starch polysaccharides
and
cellulose
, built from elementary units that are monosaccharide (
glucose
) residues.
Leather, wool, cotton, silk - all these are natural polymers.
Starch
Starch
is formed as a result of photosynthesis in the leaves of plants, and is stored in tubers, roots, and grains.
Starch
– white (granular under a microscope) powder, insoluble in cold water, in hot water it swells, forming a colloidal solution (starch paste).
Starch
is a mixture of two polysaccharides, built from amylose (10-20%) and amylopectin (80-90%).
Glycogen
Glycogen
– a polymer based on the monomer maltose.
In animal organisms, glycogen is a structural and functional analogue of plant starch.
Glycogen
is the main form of glucose storage in animal cells.
Glycogen
forms an energy reserve that can be quickly mobilized if necessary to compensate for a sudden lack of glucose.
The structure of glycogen is similar, but has even greater chain branching.
Cellulose
Cellulose
(or fiber) is the most common plant polysaccharide. It has great mechanical strength and acts as a supporting material for plants.
The purest natural cellulose
– cotton fiber – contains 85-90% cellulose. Coniferous wood contains about 50% cellulose.
Squirrels
Squirrels
– polymers, the elementary units of which are amino acid residues.
Tens, hundreds and thousands of amino acid molecules forming giant protein molecules combine with each other, releasing water due to carboxyl and amino groups. The structure of such a molecule can be represented as follows:
Squirrels
– natural high-molecular nitrogen-containing organic compounds. They play a primary role in all life processes and are carriers of life. Proteins are found in all tissues of organisms, in the blood, in the bones.
Squirrels
found in all tissues of organisms, in the blood, in the bones. Enzymes (enzymes), many hormones are complex proteins.
Protein
, like carbohydrates and fats, are the most important necessary part of food.
Natural rubber
Natural (natural) rubber
– polymer based on
isoprene
.
Natural rubber
found in the milky sap of rubber plants, mainly tropical (for example, the Brazilian Hevea tree).
Another natural product is gutta percha
– is also a polymer of isoprene, but with a different molecular configuration.
Raw rubber is sticky and fragile, and becomes brittle when the temperature drops slightly.
To give products made from rubber the necessary strength and elasticity, the rubber is vulcanized.
- sulfur is introduced into it and then heated.
Vulcanized rubber is called rubber
.
Synthetic polymers
Synthetic polymers
- These are a variety of materials, usually obtained from cheap and accessible raw materials. On their basis, plastics (plastics), artificial and synthetic fibers, etc. are obtained.
Plastics
– complex compositions into which various fillers and additives are introduced, giving the polymers the necessary set of technical properties.
Polymers and plastics
based on them, they are valuable substitutes for many natural materials (metal, wood, leather, adhesives, etc.).
Synthetic fibers
successfully replace natural ones - silk, wool, cotton.
It is important to emphasize that in a number of properties, materials based on synthetic polymers are often superior to natural ones. It is possible to obtain plastics, fibers and other compounds with a set of specified technical properties. This allows us to solve many problems of modern technology that could not be solved using only natural materials.
Polymerization resins
Polymerization resins include polymers obtained by the polymerization reaction of predominantly ethylene hydrocarbons or their derivatives.
Examples of polymerization resins: polyethylene, polypropylene, polystyrene, polyvinyl chloride, etc.
Polyethylene.
Polyethylene
– a polymer formed during the polymerization of ethylene.
Or in short:
Polyethylene
– a saturated hydrocarbon with a molecular weight from 10,000 to 400,000. It is colorless, translucent in thin layers and white in thick layers.
Polyethylene
is a waxy but hard material with a melting point of 110-125 degrees C. It has high chemical resistance and water resistance, low gas permeability.
It is used as an electrical insulating material, as well as for the production of films used as packaging material, dishes, hoses, etc.
The properties of polyethylene depend on the method of its production. High pressure polyethylene
has a lower density and lower molecular weight (10,000-45,000) than
low-density polyethylene
(molecular weight 70,000-400,000), which affects the technical properties.
Only high-density polyethylene is allowed for contact with food, since low-density polyethylene may contain catalyst residues - heavy metal compounds harmful to human health.
Polypropylene.
Polypropylene
– a polymer of propylene, a homologue of unsaturated ethylene hydrocarbons following ethylene.
In appearance it is a rubber-like mass, more or less hard and elastic.
It differs from polyethylene in its higher melting point.
Polypropylene
used for electrical insulation, for the manufacture of protective films, hose pipes, gears, instrument parts, high-strength and chemically resistant fiber. The latter is used in the production of ropes, fishing nets, etc.
Polypropylene films
much more transparent and stronger than polyethylene. Food products in polypropylene packaging can be subjected to heat treatment (cooking and heating, etc.).
Polystyrene
Polystyrene
formed during the polymerization of styrene:
It can be obtained in the form of a transparent glassy mass.
It is used as organic glass for the manufacture of industrial goods (buttons, combs, etc.).
Artificial rubber
The lack of natural rubber in our country has necessitated the development of an artificial method for producing this important material. Soviet chemists found and for the first time in the world (1928-1930) implemented on an industrial scale a method for producing synthetic rubber.
The starting material for the production of synthetic rubber is the unsaturated hydrocarbon butadiene
or divinyl, which polymerizes like isoprene.
The original butadiene is obtained from ethyl alcohol or butane, associated petroleum gas.
Condensation resins
To condensation resins
include polymers obtained by polycondensation reaction. For example:
- phenol-formaldehyde resins,
- polyester resins,
- polyamide resins, etc.
Phenol-formaldehyde resins
These high molecular weight compounds are formed as a result of the interaction of phenol ( C6H5OH
) with formaldehyde (
CH2=O
) in the presence of acids or alkalis as catalysts.
Phenol-formaldehyde resins
have a remarkable property: when heated, they first soften, and with further heating they harden.
phenolic plastics - are prepared from these resins.
. Resins are mixed with various fillers (wood flour, shredded paper, asbestos, graphite, etc.), with plasticizers, dyes, and various products are made from the resulting mass by hot pressing.
Polyester resins
An example of such resins is the polycondensation product of dibasic aromatic terephthalic acid
with dihydric alcohol
ethylene glycol
.
The result is polyethylene terephthalate
- a polymer in the molecules of which the ester group is repeated many times.
In our country, this resin is produced under the name lavsan
(abroad – terylene, dacron).
It is used to make a fiber that resembles wool, but is much stronger, producing wrinkle-resistant fabrics.
Lavsan
has high thermal, moisture and light resistance, and is resistant to alkalis, acids and oxidizing agents.
Polyamide resins
Polymers of this type are synthetic analogs of proteins. Their chains contain the same, as in proteins, repeating amide –CO–NH–
groups.
In chains of protein molecules they are separated by a link of one C
atom, in synthetic polyamides - by a chain of four or more
C
atoms.
Fibers obtained from synthetic resins - nylon
,
enant
and
anide
- in some properties they significantly exceed natural silk.
They produce beautiful, durable fabrics and knitwear. In technology, ropes made of nylon or anide are used, which are characterized by high strength. These polymers are also used as the basis for car tires, for the manufacture of nets, and various technical products.
Capron
is a polycondensate
of aminocaproic acid
containing a chain of six carbon atoms:
Enant
– aminoenanthic acid polycondensate containing a chain of seven carbon atoms.
Anid
(
nylon
and
perlon
) is obtained by the polycondensation of dibasic adipic acid
HOOC-(CH2)4-COOH
and hexamethylenediamine
NH2-(CH2)6-NH2
.