The information presented in this article will be useful for materials scientists, technologists and engineers involved in the processing and study of the properties of metals. The production of decorative coatings is increasingly being used in various branches of mechanical engineering, the automotive industry, and in the production of household items. Let's consider one of the most popular methods of creating a film - oxidation of stainless steel.
Terms, definitions, types of stainless steel oxidation
By definition, this term refers to the creation of a film of oxides on the surface of stainless steel due to redox reactions. In addition to the protective function and decorative finishing, the use of this process is involved when it is necessary to create dielectric layers and change surface physical processes occurring in an environment of high magnetic and electric fields. Depending on how the oxidation was obtained, they are distinguished:
- Thermal process
- Chemical exposure
- Electrochemical process
- Plasma oxidation
Let's look at these processes in more detail.
How does stainless steel corrode?
There are five main processes leading to corrosion of stainless steel: Homogeneous corrosion; Intergranular corrosion; Galvanic or normal corrosion, including pitting and crack corrosion; Corrosion in cracks from mechanical impact; and Microbiologically Induced Corrosion (MIC). In addition, a number of mechanical processes enhance the five basic processes of rust formation. These processes include erosion, pore formation, abrasion (flaking), formation of corrosive elements, and surface changes due to thermal or electrical influence. All these processes have one thing in common: the chromium oxide passivation layer is broken, and the unprotected iron component is oxidized. To understand the phenomenon of rusting, we will consider only two processes: Homogeneous or ordinary corrosion and pitting corrosion along with erosion, pitting and the formation of corrosive elements.
Thermal process
The thermal oxidation process involves treating metal at certain temperatures in an environment of oxygen or water vapor. Low-alloy steels and iron, when exposed to this effect, obtain a film called bluing. The temperature for bluing is 300-350 degrees Celsius. For high-alloy and high-chromium steels, this figure increases to 700 degrees.
Interesting information: bluing stainless steel at home is quite possible for anyone. There are several ways to do this. Any method must be preceded by grinding the metal and degreasing it. Dip the part or product into oil. It can be olive, machine, or best of all, weapon. After making sure that the application is even and that the oil gets into hard-to-reach places, we remove the workpiece and let the oil drain. After this, we begin heating with a blowtorch. The lower the heating rate, the higher the probability of complete removal of light fractions and obtaining a uniform bluing layer. After overcoming the threshold of 400 degrees, a characteristic black color appears on the surface. When finished, polish with soft felts and paste.
Chemical oxidation
Chemical oxidation is the production of a protective film through the interaction of metal and melts, often solutions, of oxidizing substances. The advantages of this method include:
- relative simplicity
- absence of high temperature sources
- simplicity of equipment
- low labor costs
The only and most significant disadvantage of this method is the low protective characteristics of such a film and low resistance to mechanical stress. The primary use of chemical oxidation is to apply a touch-up layer, as well as to preserve mechanisms and parts when stored in production workshops and heated warehouses.
Electrochemical oxidation
Electrochemical oxidation of stainless steels is a method that is widely used in industry. It consists in the fact that the parts are suspended on special holders. Using this device, they are lowered into a solution with alkali, after which the bath in which it is located is connected to the negative cathode. The parts are connected to the positive anode. When direct current is passed, according to a physics course, electrolysis processes occur, accompanied by an increase in temperature. The speed of application and the thickness of the resulting film depends on many factors. Main influencing factors:
- Density of flowing current.
- Electrical conductivity of the solution in which the parts are placed
- Electrolyte temperature
- Geometry and part configuration
Interesting fact: at the end of the previous decade, one of the giant Japanese automakers used galvanic blackening of stainless steel on the body of their cars. Research has shown that in the area where the roof bends into the pillars, the oxidized layer was of insufficient thickness. Long-term research into changing the place where the electrodes are attached to the body, changing the current density, and changing the exposure time did not lead to the expected result. Only by changing the electrical conductivity of the alkaline solution and adding special additives did the layer become uniform and sufficient for the quality level of the enterprise.
Complex geometry, sharp corners, curved shapes in the contours of the part lead to differences in potentials arising on the surface of stainless steel and, accordingly, lead to differences in film thickness. For such parts, it is advisable to use the previous oxidation method.
Where does corrosion occur?
Corrosion can occur in pure water, ultrapure water, steam, purified drinking water or untreated process water. To date, five processes have been identified.
Iron contamination
Joining stainless steel to carbon steel will result in iron being drawn onto the surfaces, which will be susceptible to rust when put into service. Welding temporary fasteners made of carbon steel to stainless steel and then grinding the seams results in abrasion of the chrome layer, which will corrode during operation of the system. Using carbon steel wire brushes or grinding wheels contaminated with carbon steel will cause rust. The mechanism of rust formation is very simple:
IRON + WATER + RUST,
The best means of preventing rust formation is dictated by common sense: always cover all carbon iron surfaces with wood, plastic or cardboard to avoid contact with stainless steel; never weld carbon steel to stainless steel; Always use brushes made exclusively from stainless steel and grinding wheels “designed exclusively for stainless steel”; Always perform chemical passivation with nitric or citric acid before commissioning.
Rust can cause pitting or pitting of rust on stainless steel when exposed to an oxidizing agent, so it must be removed. Therefore, passivation is necessary, which not only increases the presence of chromium (relative to iron on the surface), but also prevents any contamination with iron. There are two main technical regulations for cleaning and passivation: ASTM A 380 Standard Conditions for Cleaning, Descaling, and Passivation of Stainless Steel Parts, Equipment, and Systems and ASTM A 967 Standard Conditions for Processing Chemical Passivation of Stainless Steel Parts. . Both treated and untreated water can cause rusting (even softened water). The reason is the water content - primarily iron bicarbonates. Softening does not remove anions such as carbonates, bicarbonates, sulfates, chlorides, etc., but only exchanges cations such as calcium and magnesium with soda and potassium. Unlike iron carbonate, iron bicarbonate is completely soluble, but easily oxidizes to iron carbonate. Iron carbonate is insoluble and has a brownish-brown color. It dissolves in strong acids.
Treated or potable (potable) water is usually treated to remove suspended solids, filtered to remove fine particles and, when killed by chlorine or chlorine dioxide, bacteria. This process has little or no effect on bicarbonate ions as long as it is balanced by the low iron carbon content in the pipeline and the oxygen content. When water enters an interior environment such as stainless steel or porcelain, bicarbonates begin to oxidize:
2Fe(HCO3)2 + Ca(HCO3)2 + Cl ® 2Fe(OH)3_+ CaCl2 + 4CO2 2Fe(OH)3 ® Fe203 + H2
Iron oxide Fe203 turns brown and when this happens it is called red ironstone. The weld begins to corrode due to brown deposits due to the formation of corrosive elements under the influence of rust and calcium chloride. In untreated water, a similar reaction occurs, except for the presence of chlorine, and oxygen dissolved in the water, which is the active reagent.
6Fe(HCO3)2 O2®2Fe2(CO)3_+2Fe (OH)2 + 4H2O
Iron carbonate begins to be present and iron hydroxide forms a jelly-like substance that appears as iron oxides. There is a slight color deviation due to... Iron hydroxide is yellow. In large reservoirs, the brownest sediments are usually at the top and thin out toward the bottom. It is quite common to observe a relatively clean condition of a large tank.
Pure and highly purified water
Pure and highly purified water is typically used in industries where the result of insufficient purity can have significant consequences, such as pharmaceutical or semiconductor manufacturing. In pharmaceuticals it is called WDI or water for injection. Typical treatment involves filtration, softening, cation and ion exchange, reverse osmosis, ultraviolet treatment and, if necessary, ionization. The distillation process can be used as a final purification. The result is water with extremely low conductivity.
Type 316L stainless steel is a common material of equipment construction. Some of these complexes remain clean, but some others rust. Even systems that have been electropolished and have a surface finish of less than 10 microinches (<10 m-in Ra)
may corrode. When working with hot, highly pure steam, such systems can become black, sometimes shiny black, sometimes black with a powdery coating effect. We have received isolated sections of rust-damaged stainless piping from many different clean water and steam systems. The rust layers were examined by fluoroscopy (XPS), energy spray spectroscopy (EDS) and scanning electron microscopy (SEM). Scanning electron microscopy made it possible to visually examine the surface, energy spray spectroscopy made it possible to analyze surface anomalies with glasses, and fluoroscopy made it possible to perform a layer-by-layer analysis of rust and identify features of the molecular structure. Detailed research reports are given in (1,2). This study made it possible to classify the process of rust formation in pure and highly pure water and steam into classes I, II and III.
Rust Class I
Class 1 rust comes from external sources. Rust particles are deposited on the surface of stainless steel and, in the early stages of deposition, can be easily removed by washing. The passivation layer of stainless steel under the rust remains unchanged from the originally installed system. Rust particles usually have the same composition as the material from which the particles originate (certainly not the particles originating from corroding stainless steel). The concentration of rust is highest near the water supply and decreases further away from it. The color of the rust can vary depending on the distance from the source, from orange to russet and bright red in certain areas. Scanning electron microscopy (SEM) Class 1 corrosion appears as individual particles. The color is determined by the presence of various iron oxides and hydroxides. Orange oxide is the lowest degree of iron hydroxide, which is formed if oxygen and water are present simultaneously:
2FeO+4H2O ®2Fe(OH) + 3H2
2Fe(OH) ® Fe2O3 + H2O.
Exterior rust can come from many sources. The most likely parts are carbon iron, such as tie rods, nuts, bolts, clamps, etc. The larger the source, the more rust can form.
Pumps are the most suspect in the cleaning system. The most obvious causes of rust caused by pumps are pitting and erosion caused by uneven rotation of the suction wheel (impeller). Pitting is usually caused by improper water supply to the pump, improper pump selection, or excessive throttling during pumping. The air bubbles impact the pump working surfaces and result in a sudden shock wave that removes small particles of stainless steel. When the particle is released into the water stream, it bonds to the stainless steel pipe through electrostatic attraction. Since the surface of the particle is not passivated, it immediately begins to oxidize and rust.
Another possible process is impeller erosion. Any material has a critical rotation value, beyond which erosion increases (3). For a low alloy stainless steel alloy, this amount of rotation is about 100 feet per second. The degree of erosion varies depending on temperature. Type 304 stainless steel has a constant rate of erosion up to 300°C, and then it increases rapidly. There are no specific data available for highly purified water for various alloys.
The metallurgical properties of the impeller appear to influence the metal removal rate of the water. When 18-8 stainless steel crystallizes, two metallurgical phases occur: austenite and delta ferrite. Delta ferrite formation depends on the composition of the alloy and, if it is less than 8%, it can easily dissolve when exposed to heat. Cast pump blades typically have a higher delta ferrite content because more silicon is added during casting to ensure the fluidity of the steel when cast. This means that heat exposure will not dissolve all of the delta ferrite. The reason delta ferrite is more problematic is that it is more susceptible to erosion than austenite and contains more iron.
Rust Class II
This class of rust occurs in the presence of chlorides or other halides. This is corrosion that forms and forms on the surface of stainless steel in places where the passivation layer is damaged. This type of rust is an integral part of the surface. It most often appears on unpassivated or mechanically polished surfaces and may indicate pitting corrosion. The stainless steel underneath these pockmarks is usually very worn and may be pitted. When the composition of this rust is analyzed, the presence of chlorides or other halides is usually detected. Rust cannot be removed by mechanical means other than grinding and polishing, but acid etching is most often used. Citric acid is a good cleaning agent that will passivate stainless steel, but the presence of chlorides will cause the surface to rust again.
Class II rust is formed by a two-step reaction: the first is the dissolution of the chromium oxide passivation layer, and the second is the oxidation of iron in the material:
Cr2O3 + 10Cl-+ 2H2O ® 2CrCl3 + 4 HClO 2Fe + 3ClO- ® Fe2O3 + 3Cl-
This reaction is self-sustaining by reacting chlorine with chromium to produce hypochlorous acid as a by-product, and the hypochlorous acid oxidizes the iron and produces more chloride.
Increasing the molybdenum content of stainless steel increases its resistance to chloride attack. Similarly, replacing iron in stainless steel with nickel improves its resistance to corrosion. Progression of alloys with increasing resistance to chloride: type 304L (lowest), type 316L, type 317L, type 304LM, Alloy 625, Alloys C-276 and C 22 (highest). Any contact of stainless steel with acid chloride poses a risk of rust formation. A pH hardness >7 has a lower chance of rust formation than a pH hardness <7. Even short-term exposure to acid chloride can be the starting stage of rusting, especially if the surface of the stainless steel is rough. Mechanically polished surfaces are worse than electropolished surfaces, since microscopic pitting remains during polishing operations. Electropolishing removes these pits and produces a passivation layer with a higher Cr:Fe ratio. The pitting creates corrosion elements where acid chloride solutions can concentrate and continue to react, even if the entire system is equipped with a high-hardness water flush. The use of strong surfactants in the wash solution will aid in the removal of chloride.
Rust class III
This rust is black, not brown, and is formed in the presence of high temperature steam. When initially formed, it is blue and then turns black as it grows to an extreme thickness that prevents further penetration of oxygen. It can be found in high purity steam systems operating at high temperatures. On electropolished stainless steel surfaces, this rust is shiny black, but on non-passivated mechanically polished surfaces it can be matte black. Rust of this class on an electropolished surface forms octahedral crystals that completely cover the surface. Analysis using X-ray photoelectron spectroscopy shows that the layer is iron sesquioxide, commonly referred to as magnetic iron ore. It will not be removed by regular cleaning, but can be removed with chemicals or sanding. If the rust is shiny black, it can be left as it is quite stable. The matte rust layer may be removed and may require cleaning. After dry cleaning, usually using hot oxalic acid, the surface must be chemically passivated. When the system is subsequently put into operation, it may turn black again, but, hopefully, without the formation of a dull rusty coating.
This type of rust is the product of steam reacting at high temperatures with iron in stainless steel, resulting in the formation of magnetic iron ore. The reaction occurs in two stages:
3Fe0 + 4H2O ® FeO + Fe2)3 + 4H2 FeO + Fe2O3 ® Fe3O4
Some of the iron oxide can be replaced by nickel oxide, but iron sesquioxide will determine the color of the coating.
Plasma oxidation
Plasma oxidation occurs under conditions similar to galvanic blackening. When a certain critical value of polarization is reached, a plasma microdischarge occurs on the surface of the anodized part. Unlike electrochemical nitriding, not only the alkali solution, but also the cathode material participates in the formation of the resulting film. A characteristic feature of the presented method is deep penetration into the layer of stainless metal and the ability to obtain a uniform coating on objects of complex geometric configuration.
An interesting fact: at the point of spark breakdown during plasma oxidation, the temperature is about 10,000 K, and the pressure is comparable to 102 MPa. After the spark ceases, a sharp cooling of the surface occurs, which leads to the emergence of new physical properties and their study as elements of nanotechnology.
The coatings that are formed when using this method are characterized by increased adhesion to the base and properties close to ceramics. Considering the price of the equipment and the lack of research in this area, its labor intensity and the need for highly qualified personnel do not allow this process to be widely used in industry, being limited to expensive industries and piece products. For aluminum, titanium and magnesium alloy, plasma oxidation is finding niches and widespread use in industry.
Where to buy quality stainless steel?
is engaged in the production of high-quality corrosion-resistant stainless steel, represented by sheets, pipes, tapes, and rolls. The types of corrosion-resistant austenitic stainless steel grades presented in the assortment are widely used in various areas where there is aggressive exposure to acidic environments:
- food industry;
- medical industry;
- mechanical engineering;
- automotive industry;
- construction.
Only the right choice of stainless steel with high quality alloying and compliance with GOST will help avoid problems of corrosion of important parts.
Difficulties of blackening work associated with stainless steel
All the methods described above are ideal for ferrous alloys and low alloy steels. A special approach is required, a set of measures for blackening stainless steel as a conditionally inert alloy. The scattered data in the literature on direct blackening of stainless steel is contradictory and does not always work in practice. On a production scale, it is customary to solve this issue with a two-step approach. The first stage is anodizing stainless steel with another metal that is more prone to oxidation. This is mainly nickel, less often copper. The second stage is oxidation of the resulting surface. Chemists in many countries are developing special passivating pastes and compositions for blackening stainless steels that can induce them to oxidize.
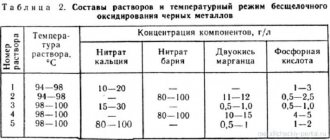
To apply a decorative film that does not work when exposed to temperature changes, on a surface that does not experience large mechanical loads, the following oxidation method can be used:
- Etching in 10% oxalic acid solution
- Washing and processing in 1% sodium sulfide solution to the required degree of blackening
- Oiling a stainless steel sample.
Based on the information presented, we can conclude that the use of blackening for stainless steel is in the nature of a commercial decorative coating. The use of oxidation to achieve higher metal characteristics is not justified and cannot be guaranteed. To obtain protective films that expand the scope of application of stainless steels, it is worth considering other methods and methods.