Special steels: types, alloying impurities
To give steel special qualities, special impurities are used, which are called alloying elements. They are introduced into the alloy composition during the smelting process when certain conditions are created. Nickel, chromium, titanium, cobalt, molybdenum, aluminum and others are used as such substances. As a result, chromium-nickel, manganese, cobalt, titanium steels and the like are obtained. For carbon steels, manganese and silicon are mainly used, since it is these components in the required proportions that impart the desired properties to such alloys.
Classification
The main parameters for classifying special steels is their structure. For such materials, the critical points are shifted downward, and therefore, when slowly cooled in air, they can acquire additional qualities. Based on this, they were divided into four classes.
Martensitic steels
The structure of such materials is needle-shaped and consists of martensite, which implies a carbon content of at least 0.15%, chromium of about 11-17% and a number of additional components in the form of vanadium, nickel, tungsten, molybdenum. It predominates in many pure and hardened metals. In this case, the martensitic component includes a carbon solution of iron in the form of a crystal lattice, which has a nonequilibrium structure. This is why martensitic steels have significant internal stress. These materials include alloys under the following brands:
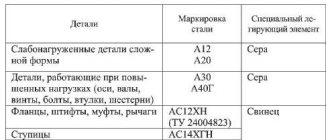
- 20Х13 – contains 12-14% chromium, up to 1% manganese and silicon, 0.16-0.25% carbon (nickel alloying is not possible);
- 10Х12НДЛ – characterized by a high nickel content (up to 1.5%);
- 18Х11МНФБ – composition includes molybdenum up to 1.1%, chromium 11.5%, carbon 0.8%, nickel 1%;
- 10Kh9MFB, 12Kh11V2MF, 13Kh11N2V2MF and 15Kh11MF are alloyed with molybdenum and vanadium in different proportions.
All of these materials are characterized by high hardness, corrosion resistance, heat resistance, the ability to self-harden, hydrogen resistance and low ductility. But with such indicators they are quite fragile. In this regard, cutting and welding them is quite difficult.
Pearlitic steels
Such special types of steels are classified as low or medium alloy steels. They contain pearlite and ferrite. Moreover, both components are alloyed with chromium. As a result, the material is highly resistant to cold brittleness.
In addition, the cooling rate affects the initial qualities of the alloy. When it changes, pearlite can acquire various transition structures. But a lot depends on what alloying impurities the steel contains. Some may help improve strength, toughness and heat sensitivity.
Pearlitic steels include 12МХ, 15ХМ, 12Х1МФ, 20ХМ, 25Х1МФ, 25Х2М1Ф, 18Х3МВ, 20Х3МВФ. All materials can be hardened, but at different temperatures.
Austenitic steels
Alloys of this nature are distinguished by the fact that they have the largest amount of impurities. As a result, they retain the austenite structure at any cooling rate. To strengthen them, they do not resort to heat treatment. However, they may have different characteristics. With a chromium content of 12-18%, corrosion resistance increases, and with 17-25%, cold resistance increases. Also, with the help of impurities, you can change the heat resistance and heat resistance indicators.
In general, austenitic steels have high toughness, good density and high resistance to mechanical stress. Among the negative aspects, it is worth highlighting the difficulty of processing with a cutter.
The list of special alloys of this class is quite extensive, since it includes high-nickel, manganese, chromium-nickel, chromium-nickel-manganese, metastable and other alloys.
Carbide steels
Carbide class alloys contain significant amounts of carbon, chromium, molybdenum, tungsten and vanadium. All these components contribute to the formation of a strong austenitic matrix and stable carbides. During crystallization from the liquid state, which results in a decrease in the dissolution of carbon in austenite, ledeburite is formed in the alloy. It is capable of maintaining high hardness at significant temperatures, and therefore is widely used for the manufacture of tools for rapid cutting of various steels. The most striking example of such steels is the material produced under the brand name R6M5. Also included in this class are chrome-tungsten, chrome-molybdenum, and high-chromium alloys.
Class I. Construction steel
Carbon steel
Construction steel is usually ordinary grade carbon steel. Construction steel is mostly supplied by metallurgical plants according to its mechanical properties (steel of group A GOST 380-60) and is used as delivered (Table 3)
Although the certificate indicates the chemical composition, it is not mandatory, except in special cases. However, at the request of the consumer, the following must be guaranteed: a) the carbon content does not exceed the upper limit specified in Table 4; b) sulfur and phosphorus content in accordance with the standards given in Table 4; c) content of chromium, nickel and copper - no more than 0.30% of each element.
In addition to the above tests, steel can, at the customer’s request, be tested for impact strength at 20°C and for cold bending and weldability.
Construction steel is supplied according to the chemical composition if the consumer requires it, if the latter subjects it to hot processing - group B steel according to GOST 380-60 (Table 4.)
Construction steel, supplied according to mechanical properties with additional requirements for chemical composition, corresponds to subgroup B.
The mechanical properties of steel must correspond to Table 3, with the exception of steel grade VSt. 3kp (section metal thickness over 40 to 100 mm), for which σt ≥ 23 kg/mm2.
The upper limits for the content of carbon, sulfur and phosphorus, as well as silicon (for mild and semi-mild steel) must correspond to Table 4. Silicon content in mild steel grade VSt. 3-0.12-0.22%, and for steel VSt. 4 and VSt. 5 - within 0.12-0.25%. The maximum content of chromium, nickel and copper is not more than 0.30% of each element, and their total content is not more than 0.60%. The arsenic content in steel is no more than 0.08%.
According to the manufacturing method, two groups of construction carbon steel should be distinguished: 1) mild steel, during the smelting process of which deoxidation operations were carried out to a fairly complete extent and the gas saturation of the steel was reduced; 2) boiling steel, less deoxidized (cheaper), when solidified, many gas bubbles are formed in the ingots, mostly brewed during hot processing.
Boiling steel has better cold deformation ability. Compared to plain steel, it accepts welding somewhat worse and is more prone to aging (blue brittleness) due to the increased content of gases in it. Therefore, it is not recommended for use in boilers, tanks and other devices operating at temperatures above 150-200°C. In addition, boiling open-hearth steel, as well as Bessemer and Thomas steel, reduces impact strength more significantly than calm open-hearth steel when the temperature drops (especially below 0°C).
Alloy steel
Alloy steel for construction purposes is not as widely used as carbon steel. However, recently the use of low alloy steel has increased dramatically. Table 5 shows some of the most well-known alloy steel compositions, and Table 6 shows its mechanical properties.
Effect of impurities on steels
Various impurities can give metals the desired characteristics. So, carbon, manganese, chromium, and molybdenum are used to increase hardness. Nickel and vanadium help improve viscosity. Manganese, silicon, and aluminum are used for shrinkage. Abrasion resistance is increased by manganese, nickel, and chlorine. Nickel, chromium, and copper provide excellent corrosion resistance. But it is important not only to combine the impurities correctly. The final characteristics largely depend on their proportions.
For example, special manganese steels must contain at least 14% of the corresponding component. When this indicator deviates, the structure of the alloy changes:
- 0.4-0.6% – martensitic;
- 10% and 12% – austenitic;
- 0.5% and 3.5% – pearlite.
However, the chlorine content remains unchanged in all three cases. In general, Mn affects thermal conductivity, so heating and cooling of such materials should be carried out with extreme caution. Products from it are produced only by casting, since cutting is very difficult. But manganese steels are well processed under pressure and do not have magnetic properties.
Another example of special steels is chromium alloy. The corresponding component is a carbide-forming component, so no more than 1% Cr is added to some steels. Even with this content, an increase in critical points is inevitable, so it is necessary to harden the material at high temperatures.
1% Cr is also found in tool alloys. In this quantity, it increases hardness and cutting characteristics.
Recently, alloying of alloys is carried out not with one component, but with several at once. In this case, it is possible to increase the influence of impurities on steel and obtain materials with special qualities. These include:
- high-speed - do not lose hardness after heating;
- wear-resistant - resistant to mechanical wear, welded after heating;
- automatic - additionally alloyed with lead, calcium and selenium, have low strength;
- spring – characterized by good elasticity, viscosity and resilience;
- construction – characterized by hardness, impact strength and elongation.
This is not the entire list of special steels. There are a great variety of them, so it is better to learn more about the composition or characteristics of a particular material from the manufacturer.
Dimensions of pearlite colonies
Annealing steel
An important characteristic of pearlite, which affects the properties of steels, is the size of the pearlite colony (Figure 3). A colony is a group of plates of cementite and ferrite that grew together, cooperatively, in austenite before colliding with other colonies
A decrease in the size of a pearlite colony is accompanied by an increase in the impact strength of steels and a decrease in their brittleness.
Increasing the brittle fracture strength of pearlite is achieved by spheroidizing cementite plates. This spheroidization can be achieved by deforming pearlite, followed by heating and holding at a temperature near the Ac1 point. Another method that provides relatively high strength and ductility to pearlite is to deform the pearlite during pearlite transformation. This leads to the formation of a polygonal structure and spheroidization of cementite.
Carbon steels of the pearlitic class.
Self-study
In the discipline "Chemical technology of coolant"
Structural materials of reactor construction and their corrosion
Purpose: To characterize the main structural materials used at nuclear power plants and consider their corrosion.
PLAN:
1. Requirements for structural materials. Characteristics of the main structural materials of nuclear power plants.
2. Stress corrosion of austenitic stainless steels.
3. Corrosion of brass.
4. Corrosion of zirconium alloys.
LITERATURE:
1. M.I. Khorsheva. Water treatment, special chemical cleaning and chemical control at nuclear power plants. Sevastopol, SYAEiP, 2000 (pp. 73-111).
2. L.A. Kulsky et al. Water in nuclear energy. Ed. L.A. Kulsky. K. Naukova thought, 1983 (pp. 43-48).
3. V.V. Goncharuk et al. Water-chemical technology of nuclear power plants and ecology. Directory. K. Naukova Dumka, 1993 (pp. 54-67).
Requirements for construction materials.
Characteristics of the main structural materials of nuclear power plants
WELDING AND WELDED MATERIALS
POROUS MATERIALS ON A METAL BASE (Tretyakov A. F.)
39.1. Classification of porous materials Porous materials (PM) on a metal basis are used as filter elements, mixers, gas lenses, noise suppressors, etc. PM are classified according to their purpose, chemical composition and type of structure-forming ...
COMPOSITE MATERIALS WITH A METAL MATRIX (Chernyshova T. A.)
38.1. Classification Composite materials are materials reinforced with fillers arranged in a certain way in the matrix. Fillers are most often substances with high energy of interatomic bonds, high strength and high modulus, but in combination ...
Heat-resistant steels
Heat resistance
— the property of a material to resist the development of plastic deformation and destruction under simultaneous exposure to an applied load and high temperatures (above 0.3 tpl) for a certain period of time.
Heat-resistant steels are used in power engineering (in the designs of gas turbine, rocket, piston and other engines), in boiler-turbine construction, and in units of the metallurgical industry.
Heat-resistant steels, parts from which are operated at temperatures up to 650 °C, are sometimes called heat-resistant
.
The simultaneous impact of two external factors on the alloy - temperature and stress - causes the development of a structural process in it called creep.
. Creep is a process of arbitrary plastic deformation at stresses lower than the short-term yield strength at a given temperature. Creep occurs at temperatures above 0.3 tmelt. The development of the creep process over time is described by the creep curve (Fig. 15.1), characterized by three stages:
1) stage of unsteady creep - the metal is deformed at an uneven decelerating rate (segment ab);
2) stage of steady creep - the metal is deformed at a constant rate (segment bс);
3) stage of destruction - the metal is deformed at an increasing speed, which ends in its destruction (segment cd).
A heat-resistant alloy part should operate only when the metal is at the stage of steady-state creep. The longer this stage at a given temperature, the longer the service life of a product made of heat-resistant material.
The reason for the development of creep is the gradual accumulation of changes in the microstructure of the material at elevated temperatures under stress: the movement of atoms and dislocations, sliding along grain boundaries. Grain boundary sliding
is a shift of grains relative to each other along common boundaries in a narrow boundary region. As a result of such sliding, discontinuities appear at the grain boundaries - pores, the accumulation of which can lead to destruction of the material.
To slow down the creep process and prolong the steady stage of creep, appropriate alloying of alloys is used:
• refractory elements that increase the recrystallization temperature;
• elements with variable solubility in the base metal, which allows for strengthening heat treatment.
Heat resistance is characterized by creep limit, long-term strength limit.
Creep limit
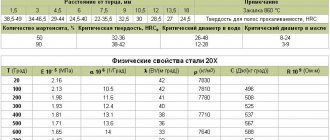
- stress under the influence of which the material is deformed by a certain amount in a certain time at a given temperature. When designating the creep limit oib/t, three numerical indices are indicated: the upper one corresponds to the test temperature value t, °C; lower - shows the specified total deformation b, %, which must be obtained in time t, h.
Long-term strength limit
- stress equal to the ratio of the load at which the sample is destroyed after a certain period of time to the original cross-sectional area. In the designation of the long-term strength limit ott, two numerical indices are given: the upper one indicates the temperature t, °C, and the lower one indicates the duration t (or base) of the test, hours. For example, o100v700, MPa, means the stress at which the sample material fails after 100 hours of testing at a temperature of 700 °C.
Heat-resistant steels are used in various fields of mechanical engineering, under different combinations of operating conditions for parts: temperature, time, load. This is due to the multivariate nature of changes in the structural-phase state of steels due to such parameters as:
• phase transformations in Fe-C, Fe-Cr-C, Fe-Cr-Ni systems;
• wide doping range;
• various options for strengthening heat treatment (hardening + tempering, isothermal hardening, hardening + aging);
• belonging to structural classes - pearlitic, martensitic, ferritic-martensitic, austenitic.
The structural class of steel is determined by the structure that the steel acquires after heating to 950 °C and cooling in air: pearlite, martensite, austenite or ferrite with martensite. Steels of all classes, except pearlitic, are highly alloyed. All steels are multicomponent alloys in which the combination of alloying elements is aimed at providing creep resistance (Fig. 15.2).
Classification of alloy steels by equilibrium structure
Classification of alloy steels by structure in the equilibrium state (by equilibrium structure) was proposed by Obergoffer and is sometimes called the Obergoffer classification
. Initially, this classification included four main classes (hypoeutectoid steels, eutectoid steels, hypereutectoid steels, ledeburite steels); was subsequently modified.
Structural classes of alloy steels
Structural classes of alloy steels
– classification characteristics of alloy steels by structure under equilibrium conditions [3].
There are hypoeutectoid steels
containing eutectoid and excess alloyed ferrite in the structure;
eutectoid steels
having a pearlitic structure, and
hypereutectoid steels
containing eutectoid and excess (secondary) carbides of the M3C type, which precipitate from austenite during cooling.
All these steels are combined into one class - pearlitic steels
.
Steels that have a ledeburite-type eutectic in their structure in the cast state are called ledeburite steels
.
With a low carbon content and a large amount of alloying element, a steel with a structure of alloyed ferrite with a certain amount of carbides is formed - ferritic class steel
.
When the steel contains a high content of an alloying element that expands the region of the γ-phase (Ni, Mn), an austenite structure is obtained, and the steel is called austenitic class steel
.
Steels in which the α γ transformation partially occurs are called semi-ferritic
and
semi-austenitic
, and their structure consists of austenite and ferrite.
Brands and characteristics
The variety of grades of pearlitic steel is not too large, there are about eight in total. Among them there are 12Х1МФ (12ХМФ), 20Х1М1Ф1ТР (EP182) and others. Today, the brand name that comes first is used. The markings in brackets are old, but may still be found here and there. It is worth noting that steel of this class with a carbon content of up to 0.35% of the total mass and with an amount of alloying elements of up to 2-5% is quite popular. The main reason for its widespread use is its low cost and relatively good mechanical qualities.
Pearlitic steel is most often used as a structural material. It is also worth noting that the weldability of steel with a carbon content of up to 0.35% and alloying elements within 3-4% is quite good.
Classification of alloy steels by structure after cooling in air
The classification of alloy steels by structure after cooling in air was proposed by the French scientist Guillet and is therefore sometimes called the Guillet classification
. This classification takes into account the structure obtained in still air of steel samples of small thickness; There are three main classes of steels:
- pearlite class;
- martensitic class;
- austenitic class.
Pearlitic steels
are characterized by a relatively low content of alloying elements,
martensitic steels are
characterized by a higher content and, finally,
austenitic steels are characterized
by a high content of alloying elements.
The classification of alloy steels by structure after cooling in air is arbitrary and applies only to the case of cooling steel samples of relatively small size in air.
Marking of alloy steels
Principles of marking alloy steels in Russia
.
The marking system for alloy steels in Russia has been developed alphanumerically, adopted in GOST standards, when each grade of alloy steel
contains a certain combination of letters and numbers. Alloying elements are designated by the following letters: X - chromium, H - nickel, B - tungsten, M - molybdenum, F - vanadium, T - titanium, Yu - aluminum, D - copper, G - manganese, C - silicon, K - cobalt, C - zirconium, B - niobium, P - boron. The letter A indicates the nitrogen content if it is in the middle of the alloy steel grade; at the end of the brand the letter A means that the steel is high quality. Numbers in steel grades indicate the content of elements according to certain existing rules. For some groups of steels, additional grade designations are accepted, based on various characteristics. More detailed information on the principles of steel marking can be found in the literature [1, 2].
Despite the fact that it is impossible to apply the GOST marking system in full for all steels, it is still the most convenient, visual, and in this sense significantly superior to the accepted steel marking system in other countries.
Prepared by: Kornienko A.E. (ICM)
Lit.:
- Gulyaev A.P. Metallurgy. - M.: Metallurgy, 1977. - UDC669.0 (075.8)
- Solntsev Yu.P., Pryakhin E.I., Voytkun F. Materials science: Textbook for universities. - M.: MISIS, 1999. - 600 p.
- Ivanov V.N. Dictionary-reference book for foundry production. – M.: Mechanical Engineering, 1990. – 384 p.: ill., ISBN 5-217-00241-1
Heat-resistant steels and alloys
Heat-resistant steel is used in the manufacture of various parts that come into contact with aggressive environments and are subject to significant loads, vibrations and high thermal effects. For example, this includes the following products: turbines, furnaces, boilers, compressors, etc. The following presents the characteristics of heat-resistant, heat-resistant alloys, classification, grades, and features of their application.
Heat-resistant steel (or scale-resistant) is a metal alloy used in an unloaded or lightly loaded state and capable of resisting gas corrosion for a long time at high temperatures (more than 550 ºС). Heat-resistant metals are products that, under high thermal influences, retain their structure, do not collapse, and are not susceptible to plastic deformation. An important characteristic of such metals is the conditional creep limit and long-term strength. Heat-resistant alloys can be heat-resistant, but they are not always so, so in aggressive environments they can quickly become damaged due to oxidation.
By application they distinguish:
Class I
— Construction steel used for construction purposes. In terms of the chemical composition, this steel is mainly carbon, and in terms of the production method, it is steel of ordinary quality (ordinary). This steel is generally not subjected to heat treatment (hardening) and is used in its pressure treated state. However, recently the possibility of strengthening this steel as a result of hardening from rolling heating has been shown.
Class II
— machine-building (structural) steel. According to the chemical composition, it is carbon or alloy steel; according to the production method, it is high-quality or high-quality. Most steel in this class is subject to heat treatment. For less critical or lightly loaded parts, bolts, wedges, blowers, shafts of low-power mechanisms, etc., cheaper steel of ordinary quality grades St.4, St.5, St.6, and St.7 are also used. In addition, steel grades St.2 and St.3 are used, used mainly for construction purposes.
Class III
— tool steel. In terms of the chemical composition, the steel is carbon and alloyed, and in terms of the production method, it is high-quality and very rarely (for the least important, for example, metalworking tools) ordinary steel. Tool steel in content and structure is mainly hypereutectoid steel, in this it differs markedly from construction and structural steel (hypoeutectoid steel). Only in special cases is tool steel used as a structural steel for machine parts for specialized purposes (ball bearings, springs). For some types of tools (for example, for hammer dies), hypoeutectoid steel is also used.
Class IV
- steel with special physical properties. According to the chemical composition, it is alloy steel, and according to the production method, it is high-quality or high-quality steel, which in some cases requires compliance with special smelting conditions (for example, in a vacuum, electroslag remelting or in an atmosphere of inert gases) and subsequent processing.
Properties of heat-resistant and heat-resistant alloys
To increase heat resistance, alloying additives are used, which also improve the strength of metals. Thanks to alloying, a protective film is formed on the surface of the alloys, which reduces the rate of oxidation of products. Main alloying elements: nickel, chromium, aluminum, silicon. During the heating process, protective oxide films (Cr,Fe)2O3, (Al,Fe)2O are formed. With a content of 5–8% chromium, the heat resistance of steel increases to 700–750 degrees Celsius, with 17% chromium – up to 1000 degrees, with 25% chromium – up to 1100 degrees.
Heat-resistant grades of metals are alloys based on iron, nickel, titanium, cobalt, strengthened by precipitation of excess phases (carbides, carbonitrides, etc.). Chromium-nickel and chromium-nickel-manganese steels have heat resistance. When exposed to high temperatures, they are not prone to creep (slow deformation under constant loads). The melting point of heat-resistant steel is 1400-1500 °C.
Classification of heat-resistant and heat-resistant alloys
At temperatures up to 300 ºС, ordinary structural (carbon) steel is used - a durable and heat-resistant metal. To work in conditions above 350 ºС, the use of heat-resistant metals is required. The main types of alloys with increased heat resistance and thermal strength:
- Pearlitic, martensitic and austenitic;
- cobalt and nickel alloys;
- refractory metals.
Pearlitic heat-resistant steels include boiler steels and silchromes containing a small percentage of carbon. The recrystallization temperature of the material increases due to alloying with molybdenum, chromium, and vanadium. The alloys are characterized by good weldability. The production of martensitic steels is carried out using pearlitic and chromium additives, hardening at 950–1100 ºС. They contain more than 0.15% carbon, 11-17% chromium, small amounts of nickel, tungsten, molybdenum, vanadium. Martensitic steels are resistant to corrosion in alkaline and acidic solutions, high humidity, and when heat treated at 1050 degrees they have high heat resistance.
Heat-resistant austenitic steels can have a homogeneous or heterogeneous structure. An alloy with a homogeneous structure that is not hardened by heat treatment contains a minimum of carbon and many alloying elements, which provides creep resistance. Such materials are suitable for use at temperatures up to 500 °C. In heterogeneous solid solutions, strengthened by heat treatment, carbide, intermetallic, and carbonitride phases are formed, which ensures the use of heat-resistant alloys under stress at temperatures up to 700 °C.
Nickel and cobalt alloys are used at temperatures up to 900 °C: they are used in the production of jet engine turbines and are the best heat-resistant materials. Cobalt alloys are slightly inferior in heat resistance to nickel alloys and are more rare. They are characterized by high thermal conductivity, corrosion resistance at high temperatures, and structural stability during long-term operation.
The nickel content in the nickel alloy is over 55%, carbon 0.06-0.12%. Depending on the structure, there are homogeneous (nichromes) and heterogeneous (nimonics) nickel alloys. Nickel-based nichromes contain chromium as an alloying additive. They are characterized not only by heat resistance, but also by high heat resistance. Nimonics consist of 20% chromium, 2% titanium, 1% aluminum. Alloy grades: KhN77TYU, KhN55VMTFKYu, KhN70MVTYUB.
At temperatures up to 1500 degrees and above, heat-resistant alloys made of refractory metals: tungsten, niobium, vanadium, etc. can work.
According to the production method, they are distinguished:
1. Ordinary quality steel
(or ordinary steel) - carbon steel with a carbon content of no more than 0.6%; it is most often smelted in large open-hearth furnaces, as well as in Bessemer and Thomas converters and poured into relatively large ingots.
The manufacturing method largely determines the composition, structure and properties of this steel. Steels of ordinary quality most often have a high content of sulfur and phosphorus, reaching 0.055-0.6% sulfur and 0.05-0.07% phosphorus in open-hearth steel, and 0.06-0.07% sulfur and 0.06-0.07% sulfur in Bessemer and Thomas steel. 0.08-0.09% phosphorus. The liquidation in this steel is often more significant than in other steel classes. Steel of ordinary quality also has an increased number of non-metallic inclusions compared to steel of the following classes. In the rolled state, steel is characterized by significant striping along the direction of metal flow. In terms of mechanical properties, ordinary steel is somewhat inferior to the steel of the following two classes: high-quality steel and high-quality steel.
According to GOST 380-60, ordinary quality steels supplied according to mechanical properties (group A) are designated St.0, St.1, St.3, St.4, St.5, St.6, St.7: supplied according to chemical composition (group B): a) open hearth - MSt.0, MSt.1, MST.4, MSt.5, Mst.6, Mst.7 and b) Bessemer - BSt.0, BSt.3, BSt.4, BSt.5, BSt.6; supplied according to chemical composition and mechanical properties (group B): Vst.1, Vst.2, etc.
2. High quality steel
- carbon or alloy steel, smelted in main open-hearth furnaces in compliance with more stringent requirements for composition, melting and casting processes. The content of sulfur and phosphorus in quality steel should not exceed (depending on the grade) 0.04% of each of these elements. The number of non-metallic inclusions is less than in ordinary quality steel.
3. High quality steel
- carbon or alloyed, most often of a complex chemical composition. Such steel is smelted in electric or acidic open-hearth furnaces of small tonnage. For high-quality steel, narrowed element content limits have been established. The content of sulfur and phosphorus in high-quality steel should not exceed 0.030 and 0.035%, respectively (for some steel grades an even lower content of these elements is established). This steel also has increased purity for non-metallic inclusions. High-quality steel is designated by the letter A, placed after the designation of grades.