Super-mirror polishing of stainless steel is the latest service in the metal processing industry. It is available only in a few regions of the country.
We have already completed orders for polishing stainless steel products for clients from St. Petersburg, Nizhny Novgorod, Kazan, Pskov, Veliky Novgorod, Moscow, Ivanovo and other Russian cities! We worked with automobile concerns, designers and design studios, and large chain stores. Each of our clients was satisfied with the polishing result.
What are the advantages of electropolishing
Electropolishing is a new technique that has already proven itself brilliantly on the market:
- Can polish different metals;
- Necessary when applying vacuum coatings to products;
- Gives better resistance to corrosion;
- This treatment gives status to the product;
- Ideal style, design, and status of the product;
- Parts can be polished in absolutely any shape;
- The minimum possible level of roughness is achieved.
Chemical methods for polishing stainless steel
It is possible to remove the thin top layer of metal and level the surface not only with the help of mechanical processing. A chemical method is also used for this - etching, that is, exposure to an aggressive chemical. The disadvantages of this method are the toxicity of the substances used, as well as the inability to achieve a mirror shine. However, etching as a polishing method also has several significant advantages:
- Rapidity. Surface treatment, unlike mechanical polishing, will require only a few minutes.
- Ability to process parts of any shape and configuration. There is no need to waste effort on manually polishing the metal.
- No power source required. Etching can be carried out in any conditions.
Acid solutions are often used as polishing agents. The initial stage of processing is carried out using sulfuric acid, and the final polishing is carried out using a nitric acid solution. Another option is to use alkaline media. They cannot level the metal, but they remove unnecessary oxide films.
A safer and more convenient option to use are special polishing pastes that contain acids and chlorides. These pastes are easy to apply due to their jelly-like consistency, but they require careful handling. The components they contain are toxic to humans. Before using them, the surface must be cleaned of dirt and degreased. The treated metal product is washed with a stream of running water to remove any remaining polishing paste.
Essence of the method
- The polishing process occurs at operating voltages of 200…350 V.
- At voltages above 200 V, a very thin (from 50 to 100 μm) vapor-gas shell is formed near the anode.
- A zone of maximum electric field strength is formed on the microprotrusions of the surface of the part.
It has been proven that the quality of electroplasma polishing depends on the operating voltage.
| Minimum voltage threshold | IN |
| Stainless steels | 220 |
| Copper and copper-based alloys (bronze, brass) | 260 |
| Aluminum alloys | 270…290 |
| Titanium alloys | 280…300 |
Processing a part using the EPP method is an ideal preparation of the surface for the subsequent application of a layer of ion-vacuum coating (titanium nitride, etc.).
Methods for polishing stainless steel
There are several technologies for polishing stainless steel, among which the most common are mechanical, chemical and their varieties. Mechanical is used to restore the mirror finish of stainless steel directly on site, as well as for workshop repairs and processing of small batches of products. When in-line processing of stainless steel parts at industrial enterprises, as a rule, the method of electropolishing in chemical solutions is used. You can bring stainless steel to a shine at home using methods and means available to everyone.
In case of minor damage or oxidation, the surface of a stainless steel product can be easily brought to a shine using polishing paste or chemical polishing reagents. If scratches and gouges on stainless steel are of a significant size, then mechanical grinding must first be performed.
Mechanical polishing
When mechanically polishing stainless steel, microprotrusions of the metal are cut off using abrasive grains. In this case, the tools used are circles, discs, rollers and tapes, and the abrasive materials are polishing pastes and suspensions. Some of them contain chemical components that, together with the abrasive, act on micro-irregularities. This type of processing is called chemical-mechanical polishing of stainless steel.
Don't miss: How to make a jointer from an electric planer with your own hands
After machining or rolling, longitudinal stripes and grooves remain on the surface of stainless steel products. These irregularities, in the best case, have a roughness class of 6–7, so grinding stainless steel to class 8–10 is a prerequisite for preparing for the polishing operation, since classes 11–14 correspond to this type of processing.
Mechanical polishing of stainless steel can be done manually, without the use of power tools or special devices. This treatment is most common in everyday life and for small volumes of repair and restoration work. The following types of production equipment are used in production plants for polishing stainless steel:
- hand-held electric and pneumatic tools;
- polishing machines;
- drum and vibration devices;
- magnetic abrasive installations.
The most common abrasive materials for polishing stainless steel are various liquid polishes, suspensions and pastes, which allow you to achieve the best results in terms of roughness. Most of them are based on technical oils, fats and substances such as paraffin and stearin, which have to be removed from the surface of the stainless steel using organic solvents.
Mechanical restoration
Polishing of stainless steel is carried out using a material represented by grains of abrasive material. When processing, a circle, disk, roller or tape is used. Various pastes, solutions and suspensions for polishing act as abrasives. The material may contain substances that, in combination with abrasive grains, remove irregularities on metal surfaces. This type of processing is called mechanical.
As a result of mechanical impacts on the metal surface, grooves and stripes with roughness up to class 7 are formed. In this case, additional refinement of stainless steel to class 10 by grinding is necessary.
Refinement of stainless steel can be done at home without the use of special devices and tools. This type of polishing is common in private workshops and garages. In industrial enterprises, the following types of tools are used:
- manual devices with electric and pneumatic drive;
- polishing and grinding machines;
- drum and vibration units;
- installations for processing using magnetic abrasive.
The following abrasive materials are used for fine grinding:
- liquid polish;
- pasta;
- suspension.
They contain mineral oils, paraffin and stearin additives as a base; they must be removed after processing with solvents.
Grinding with mechanical polishing
After damaging metal processing (cutting, welding, drilling, cleaning with hard rotary brushes, impacts), defects of various sizes are formed:
- scratches, dents;
- seams, sagging, shells;
- chips;
- cracks;
- burrs.
These surface deteriorations reduce wear resistance, reflectivity, and resistance to complex loads. To eliminate roughness and add shine to such a hard material as stainless steel, you will have to perform 4–5 operations. Grinding is carried out using an electric grinder and replaceable abrasive wheels. With a felt/felt circle, after rough cleaning, they begin to polish the product. The endless belt makes it easy to process complex stainless steel parts.
For rough grinding of stainless steel, abrasive grit 30-40, finishing 16 - 25, polishing with micropowders with grit M7 - M14, finishing to a mirror - industrial ready-made compounds (polishes).
The mechanical action of a soft circle with applied paste removes a very small amount of metal. Glossy leveling occurs due to the redistribution of the structure of the top layer of stainless steel, rather than cutting it off. Under the influence of air, active components of the paste, and heating from friction, old oxide films are destroyed and, immediately, upon cooling, new ones are created.
After mechanical polishing, ideal smoothness and, accordingly, shine in inconvenient places is not created. In this case, finish polishing by hand. Bringing a mirror finish to stainless steel by hand is a labor-intensive, time-consuming operation, but doable. The creation of a mirror begins with polishing pastes and ends with liquid polishes.
Mechanical method of polishing stainless steel
It is necessary to subject the entire visible plane to the process - partial local processing will be noticeable. Visible differences cannot be eliminated by using polish.
Electrochemical method
The technology of electrochemical polishing (ECP) of stainless steel is based on the process of movement of metal ions from the anode to the cathode. In general, such an installation consists of a metal bath with electrolyte connected to the negative pole of a direct current source (cathode). A stainless steel product is immersed in it, to which a positive potential is applied, i.e. it is the anode. When direct current is passed through the electrolyte, positive metal ions begin to detach from the surface of the stainless steel. To a greater extent, this occurs from the tops of microprotrusions, which are thus smoothed out (see figure below). The depth of metal removal during such chemical polishing of stainless steel in an electrolyte is regulated by the magnitude of the current and the duration of the process.
Don't miss: Drill for wood carving: how to choose an engraver and cutters, carving technique, video
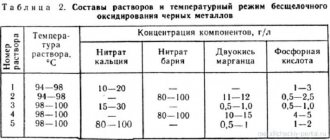
ECP allows you to process any hard-to-reach cavities and complex shaped elements by removing the same layer of metal over the entire surface of the product. Installations that perform chemical electropolishing of stainless steel operate at an electrolyte temperature of 70÷90 °C and a current density of 0.3 to 0.5 A/cm². Solutions based on a mixture of inorganic acids are used as electrolytes. For this reason, ECP is sometimes confused with chemical etching of metals and even nitric acid is mentioned in articles about them, although the main components of the electrolyte for stainless steel are phosphoric and sulfuric acids.
Chemical method
Small stainless steel parts are processed using a method that does not require much physical effort and several hours of work. Using circles can just be awkward. Immerse the cleaned workpiece in a bath with strictly dosed reagents, diluted to the required concentration with distilled water. Over a sufficient period of time, under the influence of caustic reagents, all steel roughness in contact with the liquid active medium is eliminated. Deep scratches and welding marks are first leveled with emery wheels, then smoothed with soft circles with paste of the required grain size (GOI). Otherwise, all large flaws will also be polished while maintaining their shape.
To correctly select components and their concentration in the water mass, it is advisable to know the grade of stainless steel:
- Brand X18N9T is immersed in the following composition: acids: 230 ml sulfuric, 40 ml nitric, 70 ml hydrochloric. To 1 liter of solution add acid black dye - 6 g, wood glue - 10 g, sodium chloride - 6 g. The liquid temperature is maintained at 65-70 ° C, time 5÷30 minutes.
Other options:
- Acids in proportion to the total volume: nitric 4÷5%, orthophosphoric 20÷30%, hydrochloric 3÷4%, methyl orange - 1÷1.5%, in an aqueous solution with a temperature of 18÷25 ° C, Approximate holding time 5÷ 10 min .
- Per liter of composition the amount of acids: sulfuric 230 g, hydrochloric 660 g, orange acid dye - 25 g. Maintain a temperature of 70÷75 ° C, time 2÷3 minutes.
To complete the reaction at all points and remove the resulting products, the liquid in the container is continuously stirred. You can move the steel part.
The components are aggressive. Provide protection for the skin of the hands, face, eyes, and respiratory organs.
Chemical alignment of the line of the outer boundary of the stainless steel (polishing) occurs because the reaction is more intense on the protrusions of the profile. To prevent the accumulation of interaction products in depressions, recesses, and corners, fluid movement is forced. After washing off the chemical reagents, rub with a napkin with a small amount of polish.
Electrolytic plasma polishing
Electrolytic plasma polishing (EPP) of stainless steel is also based on the process of moving positive metal ions from the anode to the cathode. But in this case, another physical phenomenon is used - the formation of a vapor-gas plasma jacket around the anode (stainless steel product), in which the process of aligning microprotrusions on its surface occurs. Electrolyte-plasma installations operate on direct current with voltages up to 400 V and with electrolyte temperatures from 60 to 90 °C. Despite the high voltage, they operate at the same current densities as during electrochemical polishing. At the same time, they process stainless steel parts several times faster: in an industrial installation, the removal of a layer of stainless steel occurs at a speed of 3 microns/min.
Another advantage of this technology is the low cost and environmental safety of the chemicals used to prepare electrolytes. In particular, when electrolyte-plasma polishing of stainless steel products, safe solutions of ammonium salts with a concentration of 3–6% are used.
Plasma polishing
The technology differs from the electrochemical procedure in the following parameters:
- the solution is not aggressive, disposal does not require special cleaning;
- voltage is higher (220 V);
- temperature is about 100 °C.
Don't miss: Flexible shaft for an engraver, drill or screwdriver: design and purpose
The reagent used is ammonium salt with a concentration in the solution of 3.1 ÷ 6.0%. The electric current density is set at 0.35 ± 0.15 A/cm²; gas bubbles are intensively formed in the contact zone of the electrolyte with the stainless steel. Discharges occur in the vapor inside the fluidized bed, ionizing the medium. Plasma tongues appear that specifically act on the steel, polishing it. The time required for one dive is within 6 minutes, based on a power consumption of 5 Wh/cm².
For a stable process of polishing a surface of a certain area using the electroplasma method, the appropriate power of the installation is required. You cannot reduce its value in the hope of increasing the duration of treatment in the bath. The conditions for the formation of a plasma-ionized layer will not be met.
Unscrupulous mechanical preparation will be evident. Residual traces of welds, scratches, and dents cannot be hidden with polish.
Plasma polishing - difficult but effective
There is another surface treatment method based on processes in the metal when it is immersed in a solution and simultaneously exposed to high voltage. Unlike the previous method, only environmentally friendly compounds based on ammonium salts are used.
Plasma method of processing products
The essence of plasma polishing of stainless steels is as follows. The product must be a positive anode. When exposed to high voltages of more than 200 V, the electrolyte begins to boil right at the surface of the part, which leads to the formation of a thin vapor-gas shell (50–100 microns). Electric current, when passing through this film, promotes the occurrence of plasma processes. In places of microprotrusions, the electric field strength increases significantly, which leads to the occurrence of pulsed discharges.
Boiling of the electrolyte at the surface of the part
Plasma polishing removes from the product a very thin layer with a high content of foreign inclusions. As a result, the surface has a mirror shine and has high adhesive properties. In addition, this method combines three operations at once: degreasing, etching and surface activation. However, to achieve the desired result, the surface of the product must be carefully prepared. Any defects, risks, scratches, etc. after such treatment will not be eliminated, but, on the contrary, will become even more noticeable. Therefore, preliminary rough manual polishing cannot be avoided.
Why is electropolishing better than regular polishing?
In addition to the visual effect, electrolytic plasma polishing outperforms mechanical polishing in terms of the final characteristics of the product and its processing.-
Technical characteristics of the surface after treatment:
A minimum surface roughness of R=0.03…0.02 µm is achieved. The surface cleanliness class is increased to maximum 14 (mirror polishing).- Polishing removes burrs up to 0.3 mm in height.
- The use of EPP cleans the surface of the part from inclusions of abrasives.
- Electric pulse polishing removes the effects of welding from the surface - tarnished color.
- Improves surface resistance to metal corrosion
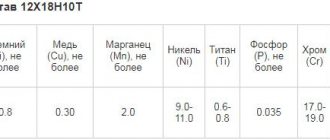
Within a few minutes of processing, the part acquires a mirror shine. The technique has been developed for the use of electrolyte-pulse polishing of parts made of stainless steel, copper-based alloys (brasses and bronzes of various compositions), aluminum, titanium - bringing the surface to a mirror shine. In relation to chromium steels of the stainless class, grades 201, 304, 316, 321 according to the AISI classification (from 08Х18Н10 to 12Х18Н10Т, 12Х15Г9НД), the more chromium in stainless steel, the better the “mirror effect”.
Experimental processing
Methodology for experimental study of polishing steel X18N10T using the electrolyte-plasma method
To study the characteristics of the installation and develop the methodology for studying the processes of electrolytic plasma polishing, a study was carried out of the patterns of polishing austenitic stainless steel X18N10T in solutions of ammonium sulfate of different concentrations.
Metal plates 1 mm thick were used in the experiments.
Current values were measured with an accuracy of ± 0.05 A, and voltages of ± 2 V. The electrolyte temperature during the experiment was maintained with an accuracy of ± 1°C, which is quite sufficient for studying the basic laws of the process and testing the technology. To study metal removal during polishing, samples were weighed before and after polishing with an accuracy of ±0.00005 g, and the mass difference (Dm) was estimated.
Current-voltage characteristics were measured at temperatures of 70, 75, 80 and 85°C and electrolyte concentrations of 3, 4, 5 and 6%, that is, in the range of parameter values used in industrial installations. In parallel, the specific power was assessed with the same parameters.
The current-voltage characteristics were measured starting from high voltages, at which a breakdown of the PPO began to be observed, recorded by sharp surges of current through the sample.
Electroplasma metal polishing
One type of leveling treatment can be called electroplasma polishing. Under the influence of electric current, a plasma cloud is formed around the workpiece; for this purpose, PPPs are used - plasma polishing units. As a result, a thin top layer is removed, its thickness does not exceed several microns.
Electroplasma treatment has several advantages:
- The surface is given a pleasant mirror shine.
- Small burrs are removed from metal products and the surface becomes smooth.
- Excessive sharpness of the edges is removed, they become safe to touch.
Using the UPP, you can process not only stainless steel products, but also parts made of copper and titanium alloys.
offers various types of processed stainless steel metal products. Mechanical, chemical and electrochemical processing make it possible to create parts with high surface quality; it becomes perfectly smooth. A wide selection of rolled metal allows you to choose everything you need to solve various problems. To order rolled metal and get exactly what you need, get detailed advice from our specialists.
Electrochemical polishing of stainless steel
Another popular surface treatment method for stainless steel is the electrolytic process. This polishing is carried out according to the following principle: the part is immersed in an electrolyte and connected to a current source. The part plays the role of an anode; a special conductive plate is used as a cathode. An electric current is passed through the system, as a result of which the top layer of the surface begins to selectively dissolve and level out.
If the temperature of the electrolyte and the current increase, polishing occurs more intensely, resulting in a thicker layer of metal being removed. The treated surface allows further application of additional galvanic coating. It becomes perfectly smooth and acquires a pleasant shine.