Table of hardness of metals according to the Mohs scale:
| Hardness | Metal |
| 0.2 | Cesium |
| 0.3 | Rubidium |
| 0.4 | Potassium |
| 0.5 | Sodium |
| 0.6 | Lithium |
| 1.2 | Indium |
| 1.2 | Thallium |
| 1.25 | Barium |
| 1.5 | Strontium |
| 1.5 | Gallium |
| 1.5 | Tin |
| 1.5 | Lead |
| 1.5 | Mercury |
| 1.75 | Calcium |
| 2.0 | Cadmium |
| 2.25 | Bismuth |
| 2.5 | Magnesium |
| 2.5 | Zinc |
| 2.5 | Lanthanum |
| 2.5 | Silver |
| 2.5 | Gold |
| 2.59 | Yttrium |
| 2.75 | Aluminum |
| 3.0 | Copper |
| 3.0 | Antimony |
| 3.0 | Thorium |
| 3.17 | Scandium |
| 3.5 | Platinum |
| 3.75 | Cobalt |
| 3.75 | Palladium |
| 3.75 | Zirconium |
| 4.0 | Iron |
| 4.0 | Nickel |
| 4.0 | Hafnium |
| 4.0 | Manganese |
| 4.5 | Vanadium |
| 4.5 | Molybdenum |
| 4.5 | Rhodium |
| 4.5 | Titanium |
| 4.75 | Niobium |
| 5.0 | Iridium |
| 5.0 | Ruthenium |
| 5.0 | Tantalum |
| 5.0 | Technetium |
| 5.0 | Chromium |
| 5.5 | Beryllium |
| 5.5 | Osmium |
| 5.5 | Rhenium |
| 6.0 | Tungsten |
| 6.0 | β-Uranium |
Plasticity test
The ductility characteristics of metals are usually determined by static tests. The most revealing is the tensile test. To find out which metal is the most ductile, it is necessary to subject samples of the same size to this effect under similar temperature conditions. The amount of deformation that a metal sample can withstand before failure is an objective indicator of ductility.
The numerical expression of the tensile test result is two main coefficients. Relative elongation is the percentage ratio of the increased length of the sample after rupture caused by deformation to the original one. The most ductile metal - gold - has an indicator of 65%. For comparison: for iron it is 40-50, for aluminum it is 30-40.
The second indicator of plasticity is the relative narrowing of the cross section of the sample. For gold, the initial cross-section of the sample is 90% larger than what it had before breaking. For aluminum this figure is 80%, for copper - 75%.
Melting point table for low-melting metals and alloys:
| Metal name | Melting point, oC |
| Mercury | -38,83 |
| France | 25 |
| Cesium | 28,44 |
| Gallium | 29,7646 |
| Rubidium | 39,3 |
| Potassium | 63,5 |
| Sodium | 97,81 |
| Indium | 156,5985 |
| Lithium | 180,54 |
| Tin | 231,93 |
| Polonium | 254 |
| Bismuth | 271,3 |
| Thallium | 304 |
| Cadmium | 321,07 |
| Lead | 327,46 |
| Zinc | 419,53 |
Melting point table for medium-melting metals and alloys:
| Metal name | Melting point, oC |
| Antimony | 630,63 |
| Neptunium | 639 |
| Plutonium | 639,4 |
| Magnesium | 650 |
| Aluminum | 660,32 |
| Radium | 700 |
| Barium | 727 |
| Strontium | 777 |
| Cerium | 795 |
| Ytterbium | 824 |
| Europium | 826 |
| Calcium | 841,85 |
| Lanthanum | 920 |
| Praseodymium | 935 |
| Germanium | 938,25 |
| Silver | 961,78 |
| Neodymium | 1024 |
| Promethium | 1042 |
| Actinium | 1050 |
| Gold | 1064,18 |
| Samarium | 1072 |
| Copper | 1084,62 |
| Uranus | 1132,2 |
| Manganese | 1246 |
| Beryllium | 1287 |
| Gadolinium | 1312 |
| Terbium | 1356 |
| Dysprosium | 1407 |
| Nickel | 1455 |
| Holmium | 1461 |
| Cobalt | 1495 |
| Yttrium | 1526 |
| Erbium | 1529 |
| Iron | 1538 |
| Scandium | 1541 |
| Thulium | 1545 |
| Palladium | 1554,9 |
| Protactinium | 1568 |
Domes in Russia are covered with pure gold...
Despite the centuries-old history of gold mining, this metal has always been considered rare and precious. This is the most ductile metal. This quality makes it cost-effective to use for decorative finishing of interior elements or even for covering church domes. To cover a large area, very little precious metal is required: 1 gram of plate can be forged into a sheet with an area of 1 m 2.
Even the manual method of producing sheets for gilding makes it possible to achieve a thickness of a thousandth of a millimeter. This thickness allows the gold plates to adhere to the surface due to molecular attraction. The technology for producing tinsel has improved significantly. Now robotic lines are used to flatten gold sheets, but the process is based on the high plasticity of the source material.
Melting point table for refractory metals and alloys:
| Metal name | Melting point, oC |
| Lutetium | 1652 |
| Titanium | 1668 |
| Thorium | 1750 |
| Platinum | 1768,3 |
| Zirconium | 1855 |
| Chromium | 1907 |
| Vanadium | 1910 |
| Rhodium | 1964 |
| Technetium | 2157 |
| Hafnium | 2233 |
| Ruthenium | 2334 |
| Iridium | 2466 |
| Niobium | 2477 |
| Molybdenum | 2623 |
| Tantalum | 3017 |
| Osmium | 3033 |
| Rhenium | 3186 |
| Tungsten | 3422 |
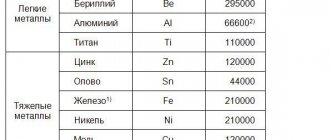
Density:
Depending on their density, metals are divided into light (density from 0.53 to 5 g/cm³) and heavy (from 5 to 22.6 g/cm³).
The lightest metal is lithium (density 0.53 g/cm³). It is currently impossible to name the heaviest metal, since the densities of osmium and iridium - the two heaviest metals - are almost equal (about 22.6 g / cm³ - exactly twice the density of lead), and it is extremely difficult to calculate their exact density: for To do this, the metals must be completely purified, because any impurities reduce their density.
What are they?
In the nomenclature of IUPAC, a world-respected international organization that oversees theory and practice in the field of chemistry, the term “light metals” is absent.
Unofficially, light metals include substances with a density of less than 5 grams per cubic centimeter.
Different lists include five to ten items.
The most common division is by use:
- On this basis, five main ones are distinguished: aluminum, beryllium, magnesium, titanium, lithium.
- They are supplemented by “exotics”: gallium, indium, bismuth, thallium, cadmium.
The second group is classified as rare metals.
These elements are called rare because in practice they are used recently and not as widely as traditional materials.
Plastic:
Most metals are ductile, meaning metal wire can be bent without breaking. This occurs due to the displacement of layers of metal atoms without breaking the bond between them.
The most ductile are gold, silver and copper. Gold can be used to make foil 0.003 mm thick, which is used for gilding products. However, not all metals are ductile. Wire made of zinc or tin crunches when bent; When deformed, manganese and bismuth hardly bend at all, but immediately break.
Plasticity also depends on the purity of the metal. Thus, very pure chromium is very ductile, but, contaminated with even minor impurities, it becomes brittle and harder. Some metals, such as gold, silver, lead, aluminum, osmium, can grow together, but this can take decades.
Methods of obtaining
The technology for smelting light metals was developed by the mid-19th century.
To obtain them in metallurgy, three methods are used:
- Electrolysis of molten salts. That is, the accumulation of dissolved components or other substances on the electrodes. The reaction is triggered by an electric current passed through a solution or molten electrolyte.
- Metallothermy. Reduction from their compounds by other, more active metals. The process takes place at elevated temperatures.
- Electrothermy. The material is heated, then melted by the heat generated from the electrical current.
The production of light elements is a very energy-intensive process. Therefore, metallurgical plants are located closer to energy sources.
Unlike heavy metals: their base enterprises are tied to the deposit.
The value of light, especially non-ferrous metals, is due to the second method of production - recycling scrap.
Electrical conductivity:
All metals conduct electricity , due to the presence in their crystal lattices of mobile electrons that move under the influence of an electric field.
Silver, copper and aluminum have the highest electrical conductivity. For this reason, the last two metals are most often used as wire materials. Sodium also has very high electrical conductivity. In experimental equipment, attempts are known to use sodium conductors in the form of thin-walled stainless steel pipes filled with sodium. Due to the low specific gravity of sodium, with equal resistance, sodium “wires” are much lighter than copper and even somewhat lighter than aluminum.
Water, fire...earth? And a little about ductile metals.
I continue the story about the work on the “Jewelry Burime” project. For those who are not aware of the project, I suggest reading two previous entries on my blog:
Now I have started working with blanks received from my main partner in the project - a wonderful jeweler Irina Vasilyeva from Moscow. And I have six blanks. And they are very, very unusual. Irina and her husband work and teach working with ductile metals in their studio Metalclaystudio. And I even managed to attend their visiting master class in St. Petersburg. And it took place in a surprisingly favorite place near my home - in the old building of the Slutskaya plant on the Kozhevennaya line of Vasilievsky Island. I already wrote about this on social networks, so whoever reads them is aware :). Everything I love: old brick, worn paint in places and most importantly - huge windows overlooking the bay, passing ships and a brand new bridge.
And these are all our baked plastic bronze blanks from the master class.
I got distracted :).
About ductile metals. I have 4 ductile bronze blanks from Irina. I won't go into details. But, briefly from the materials of the plastic metal studio Metalclaystudio.
“Metal clay is an innovative material. It was developed around 1990 in Japan by a metallurgist from Mitsubishi Materials. The material itself was plastic 999 silver clay.
By now, the material has gained recognition and enormous popularity among jewelers. In the United States, one of the largest jewelry competitions has a separate category for metal clays, officially recognized as a jewelry category.
The material consists of three main components: metal powder, organic plasticizer and distilled water.
The “alchemical process” has a very simple physical basis - it is a simplified version of the use in the civilian sector of “powder metallurgy”. The technology of powder metallurgy itself is several thousand years old - it has been known since the times of Ancient Egypt. This technology is ideal for producing complex-shaped products obtained by pressing and sintering, rather than lengthy machining, milling, etc.”
So. My third work from the “Movement and Light” series as part of the “Jewelry Burime” project is the “Chameleon” earrings, created using a ductile bronze blank in the form of a circle with the silhouette of a chameleon and a peridot eye.
I admit, for a long time I could not understand how I could figure out “movement and light” from a statically presented “animal”. And here is my reasoning, based, of course, on information gleaned from the great and huge network :).
The chameleon is one of the most unusual and beautiful lizards on the planet.
A distinctive feature of chameleons is their eyes, covered with fused eyelids with a small hole for the pupil. All-round visibility is provided by uncoordinated movements of the left and right eyes, which greatly helps in a successful hunt.
The most interesting feature of the chameleon is the change in color and pattern of the skin.
Due to the structural features of the skin layers, a light chameleon quickly turns yellow or green, then purple-red, up to dark brown or black.
Green colors additionally appear as a result of the refraction of light rays in the surface layer of the skin containing guanine crystals.
And who after this will argue with the fact that a chameleon is movement and light itself?
The result was modern, fashionable earrings. Both are different, as my daughter says :). And why should they be the same? Let us remember Hundertwasser’s words about socks. Kidding.
Bronze, brass, silver, peridot, hot enamel on copper.
Unusual earrings made of four metals: bronze, brass, copper and silver. One is a chameleon as is. The second is the eye of a chameleon. Style... hmm... safari? I see them with an animal print dress or other safari-inspired clothing. And you?
Thank you for your attention and of course I welcome your comments!