Position in the periodic table
Metals occupy groups I-II and secondary subgroups of groups III-VIII. Metallic properties, i.e. the ability to donate valence electrons or be oxidized increases from top to bottom as the number of energy levels increases. From left to right, metallic properties weaken, so the most active metals are in groups I-II, the main subgroups. These are alkali and alkaline earth metals.
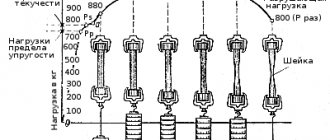
The degree of activity of metals can be determined by the electrochemical series of voltages. Metals that come before hydrogen are the most active. After hydrogen come weakly active metals that do not react with most substances.
Rice. 1. Electrochemical series of metal voltages.
Element No. 55 Cesium is the softest metal
The name “cesium” comes from the Latin caesius - “sky blue”: in the spectrum emitted by a highly heated substance, two bright blue stripes are visible in the infrared range. In its pure form, it reflects light well, looks like light gold and has a silvery-yellow color. Cesium is the softest metal in the world, the Brinell hardness index is 0.15 Mn/m2 (0.015 kgf/cm2). Melting point: +28.5°C, therefore, under normal conditions, at room temperature, cesium is in a semi-liquid state.
It is a rare, expensive and extremely reactive metal. In electronics, radio engineering and the high-tech chemical industry, cesium and alloys based on it are increasingly used and the need for it is constantly growing. Its chemical activity and ability to form compounds with the highest electrical conductivity are in demand. Cesium is an important component in the production of special optical devices, lamps with unique properties and other high-tech products. At the same time, softness is not its most sought-after quality.
Cesium abundance in nature
There is no exact data in the literature on how much cesium is available on the globe. It is only known that it is one of the rare chemical elements. It is believed that its content in the earth's crust is in any case several hundred times less than rubidium and does not exceed 7 x 10~4%.
Cesium occurs in an extremely dispersed state (on the order of thousandths of a percent) in many rocks; Minor amounts of this metal were also found in sea water. It is found in higher concentrations (up to several tenths of a percent) in some potassium and lithium minerals, mainly in lepidolite. But what is especially significant is that, unlike rubidium and most other rare elements, cesium forms its own minerals - pollucite, avogadrite and rodicite. Rodicite is extremely rare, and some authors classify it as a lithium mineral, since its composition (R20 ^AlO3-3BiO3, where R20 is the sum of alkali metal oxides) usually includes more lithium than cesium. Avogadrite (K, Cs)[BF4] is also rare, and pollucites are not common; their deposits are thin, but they contain at least 20, and sometimes up to 35%, cesium. Pollucites of CHIA (South Dakota and Maine), South-West Africa, Sweden and the Soviet Union (Kazakhstan, etc.) are of greatest practical importance.
Pollucites are aluminosilicates, complex and very strong compounds. Their composition is determined by the formula (Cs, Na) [AlSi2O6]-gaH20, and although they contain a lot of cesium, it is not so easy to extract it. To “open” the mineral and convert valuable components into soluble form, it is treated by heating with concentrated mineral acids - hydrofluoric or hydrochloric and sulfuric. Then the solution is freed from all heavy and light metals and, what is especially difficult, from cesium’s constant satellites - alkali metals: potassium, sodium and rubidium.
Modern methods for extracting cesium from pollucites are based on preliminary fusion of concentrates with excess lime and a small amount of fluorspar. If the process is carried out at 1200° C, then almost all of the cesium is sublimated in the form of Cs20 oxide. This sublimate, of course, is contaminated with admixtures of other alkali metals, but it is soluble in mineral acids, which simplifies further operations.
Cesium is extracted from lepidolites along with rubidium as a by-product of lithium production. Lepidolites are pre-fused (or spekadet) at a temperature of about 1000 ° C with gypsum or potassium sulfate and barium carbonate. Under these conditions, all alkali metals are converted into easily soluble compounds - they can be leached with hot water.
After separating the lithium, it remains to process the resulting filtrates, and here the most difficult operation is the separation of cesium from rubidium and a huge excess of potassium. As a result, some cesium salt is obtained - chloride, sulfate or carbonate. But this is only part of the story, since the cesium salt must be converted into cesium metal. To understand the complexity of the last stage, it is enough to point out that the discoverer of cesium, the great German chemist Bunsen, was never able to obtain element No. 55 in the free state. All methods suitable for the recovery of other metals did not give the desired results. Cesium metal was first obtained only 20 years later, in 1882, by the Swedish chemist Setterberg in the process of electrolysis of a molten mixture of cesium and barium cyanide, taken in a ratio of 4:1. Barium cyanide was added to lower the melting point. However, barium contaminated the final product, and it was difficult to work with cyanides due to their extreme toxicity, and the yield of cesium was very small. A more rational method was found in 1890 by the famous Russian chemist N.N. Beketov, who proposed reducing cesium hydroxide with metallic magnesium in a stream of hydrogen at elevated temperatures. Hydrogen fills the device and prevents the oxidation of cesium, which is distilled into a special receiver. However, in this case, the cesium yield does not exceed 50% of the theoretical one.
The best solution to the difficult problem of obtaining cesium metal was found in 1911 by the French chemist Axpil. With the Axpil method, which still remains the most common, cesium chloride is reduced with calcium metal in a vacuum, and the reaction 2CsCl + Ca -> CaC12 + 2Cs goes almost to completion. The process is carried out in a special device (in laboratory conditions - made of quartz or refractory glass), equipped with an extension. If the pressure in the device is not more than 0.001 mm Hg. Art., the process temperature may not exceed 675 ° C. The released cesium evaporates and is distilled into the process, and calcium chloride remains completely in the reactor, since under these conditions the volatility of the salt is negligible (the melting point of CaC12 is 773 ° C, i.e. at 100°C above the process temperature). As a result of repeated distillation in a vacuum, absolutely pure cesium metal is obtained.
Many other methods for obtaining cesium metal from its compounds are described in the literature, but, as a rule, they do not promise any special advantages. Thus, when replacing calcium metal with its carbide, the reaction temperature has to be increased to 800 ° C, and the final product is contaminated with additional impurities. It is possible to decompose cesium azide or reduce its dichromate with zirconium, but these reactions are explosive. However, when replacing dichromate with cesium chromate, the reduction process proceeds smoothly, and although the yield does not exceed 50%, very pure metallic cesium is distilled off. This method is applicable for obtaining small amounts of metal in a special vacuum device.
World production of cesium is relatively small, but recently it has been constantly increasing. One can only guess at the scale of this growth; the figures are not published.
The shiny surface of metallic cesium has a pale golden color. This is one of the most fusible metals: it melts at 28.5 ° C, boils at 705 ° C under normal conditions and at 330 ° C in a vacuum. The fusibility of cesium is combined with great lightness. Despite the rather large atomic mass (132.905) of the element, its density at 20° C is only 1.87. Cesium is many times lighter than its neighbors on the periodic table. Lanthanum, for example, having almost the same atomic mass, is more than three times more dense than cesium. Cesium is only twice as heavy as sodium, and their atomic masses are in the ratio 6:1. Apparently, the reason for this lies in the peculiar electronic structure of cesium atoms. Each of its atoms contains 55 protons, 78 neutrons and 55 electrons, but all these numerous electrons are located relatively loosely - the ionic radius of cesium is very large - 1.65 A. The ionic radius of lanthanum, for example, is only 1.22 A, although its atom contains 57 protons, 82 neutrons and 57 electrons.
The most remarkable property of cesium is its exceptionally high activity. It is superior to all other metals in its sensitivity to light. The cesium cathode emits a stream of electrons even when exposed to infrared rays with a wavelength of 0.80 microns. In addition, the maximum electron emission, which exceeds the normal photoelectric effect by hundreds of times, occurs in cesium when illuminated with green light, while in other photosensitive metals this maximum appears only when exposed to violet or ultraviolet rays.
For a long time, scientists hoped to find radioactive isotopes of cesium in nature, since rubidium and potassium have them. But in natural cesium it was not possible to detect any isotopes other than the completely stable ir;3Cs. True, 22 radioactive isotopes of cesium with atomic masses from 123 to 144 have been artificially obtained. In most cases, they are short-lived: half-lives are measured in seconds and minutes, less often - several hours or days. However, three of them do not decay so quickly - these are 134Cs, 137Cs and 135Cs, with a lifetime of 2.07; 26.6 and 3 • 106 years. All three isotopes are formed in nuclear reactors from the decay of uranium, thorium and plutonium; their removal from reactors is quite difficult.
The chemical activity of cesium is extraordinary. It reacts very quickly with oxygen and not only ignites instantly in air, but is capable of absorbing the slightest traces of oxygen in a deep vacuum. It rapidly decomposes water even at ordinary temperatures; in this case, a lot of heat is released, and the hydrogen displaced from the water immediately ignites. Cesium even reacts with ice at -116° C. Its storage requires great care.
Cesium also interacts with carbon. Only the most advanced modification of carbon—diamond—is able to withstand its “onslaught.” Liquid molten cesium and its vapor loosen soot, charcoal and even graphite, penetrating between carbon atoms and forming peculiar, rather strong compounds of golden yellow color, which in the limit, apparently, correspond to the composition C8Cs5. They ignite in air, displace hydrogen from water, and when heated, decompose and release all the absorbed cesium.
Even at ordinary temperatures, reactions of cesium with fluorine, chlorine and other halogens are accompanied by ignition, and with sulfur and phosphorus - by explosion. When heated, cesium combines with hydrogen, nitrogen and other elements, and at 300 ° C it destroys glass and porcelain. Cesium hydrides and deuterides are highly flammable in air, as well as in fluorine and chlorine atmospheres. Cesium compounds with nitrogen, boron, silicon and germanium, as well as carbon monoxide, are unstable and sometimes flammable and explosive. Cesium halides and cesium salts of most acids, on the contrary, are very strong and stable. The activity of the original cesium is manifested in them only in the good solubility of the vast majority of salts. In addition, they are easily converted into more complex complex compounds.
Cesium alloys and intermetallic compounds are always relatively low-melting.
Cesium has another very important property, closely related to its electronic structure. The fact is that it loses its single valence electron more easily than any other metal; This requires very little energy—only 3.89 eV. Therefore, producing plasma from cesium requires much less energy than using any other chemical element.
How the element was discovered
The history of cesium begins in the 19th century. Scientists (chemists Kirchhoff and Bunsen) examined the mineral springs of the Black Forest using spectral analysis and found sky-blue lines of an unknown element. The element was named after the color of the lines on the spectrogram (caesius - blue).
Geochemistry and Mineralogy
The average cesium content in the earth's crust is 3.7 g/t. There is a slight increase in cesium content from ultramafic rocks (0.1 g/t) to acidic rocks (5 g/t). The bulk of it in nature is in dispersed form and only a small part is contained in its own cesium minerals (pollucite, etc.). Consistently increased amounts of cesium are observed in morganite (1-4%), rodicite (about 5%), avogadrite and lepidolite (0.85%). In terms of crystal chemical properties, cesium is closest to rubidium, potassium and thallium. Cesium is found in higher quantities in potassium minerals. Cesium, like rubidium, tends to accumulate at later stages of magmatic processes, and its concentrations reach the highest values in pegmatites. The average cesium content in granite pegmatites is about 0.01%, and in individual pegmatite veins containing pollucite it even reaches 0.4%, which is about 40 times higher than in granites. The highest concentrations of cesium are observed in rare metal-replaced microcline-albite pegmatites with spodumene. During the pneumatolithic-hydrothermal process, increased amounts of cesium are associated with massifs of greisenized alaskites and granites with quartz-beryl-wolframite veins, where it is present mainly in muscovites and feldspars. In the hypergenesis zone (under surface conditions), cesium accumulates in small quantities in clays, clayey rocks and soils containing clay minerals, sometimes in manganese hydroxides. The maximum cesium content is only 15 g/t. The role of clay minerals is reduced to sorption; cesium is drawn into the interpacket space as an absorbed base. Active migration of this element in waters is very limited. The main amount of cesium migrates “passively”, in clay particles of river waters. In seawater, the concentration of cesium is about 0.5 μg/l. Among the cesium minerals proper, the most common are pollucite (Cs, Na)[AlSi2O6] nH2O (22–36% Cs2O), cesium beryl (pezzottaite) Be2CsAl2(Si6O18) and avogadrite (KCs)BF4. The last two minerals contain up to 7.5% cesium oxide. Among other cesium minerals, galchaite (Cs,Tl)(Hg,Cu,Zn)6(As,Sb)4S12 and margaritasite (Cs,K,H3O)2(UO2)2V2O8·H2O are also known.
Electronic circuit of cesium
Cs:
1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s1 Short notation:
Cs:
[Xe]6s1
The cesium atom and +2La, +3Ce, +4Pr have the same electronic configuration
The order of filling the shells of a cesium (Cs) atom with electrons: 1s → 2s → 2p → 3s → 3p → 4s → 3d → 4p → 5s → 4d → 5p → 6s → 4f → 5d → 6p → 7s → 5f → 6d → 7p.
The 's' sublevel can have up to 2 electrons, the 's' level can have up to 6 electrons, the 'd' level can have up to 10 electrons, and the 'f' level can have up to 14 electrons.
Cesium has 55 electrons, let's fill the electron shells in the order described above:
- 2 electrons in the 1s sublevel
- 2 electrons in the 2s sublevel
- 6 electrons in the 2p sublevel
- 2 electrons in the 3s sublevel
- 6 electrons in the 3p sublevel
- 2 electrons in the 4s sublevel
- 10 electrons per 3d sublevel
- 6 electrons in the 4p sublevel
- 2 electrons in the 5s sublevel
- 10 electrons in the 4d sublevel
- 6 electrons in the 5p sublevel
- 1 electron in the 6s sublevel
Cesium oxidation state
Cesium atoms in compounds have oxidation states of 1, -1.
The oxidation state is the conditional charge of an atom in a compound: the bond in a molecule between atoms is based on the sharing of electrons, thus, if an atom’s charge virtually increases, then the oxidation state is negative (electrons carry a negative charge), if the charge decreases, then the oxidation state is positive.
Cesium ions
1+Cs 1-Cs
Cs 0
Valency Cs
Cesium atoms in compounds exhibit valency I.
The valency of cesium characterizes the ability of the Cs atom to form chemical bonds. Valence follows from the structure of the electron shell of the atom; electrons involved in the formation of chemical compounds are called valence electrons. A more extensive definition of valency is:
The number of chemical bonds by which a given atom is connected to other atoms
Valence has no sign.
Quantum numbers Cs
Quantum numbers are determined by the last electron in the configuration; for the Cs atom these numbers have the value N = 6, L = 0, Ml = 0, Ms = ½
Isotopes
Known isotopes of cesium with mass numbers from 112 to 151 (number of protons 55, neutrons from 57 to 96), and 22 nuclear isomers. Natural cesium is a monoisotopic element consisting of a single stable isotope, 133Cs.
Decay scheme of Cs-137
The longest-lived artificial radioactive cesium nuclide is 135Cs, with a half-life T1/2 of about 2.3 million years. Another relatively long-lived isotope is 137Cs (T1/2 = 30.17 years).
Cesium-137 is one of the culprits of radioactive contamination of the biosphere, as it is formed during nuclear fission in nuclear reactors and during nuclear weapons testing. Cesium-137 undergoes beta decay, the daughter isotope is stable barium-137.
Ionization energy
The closer the electron is to the center of the atom, the more energy is needed to tear it off. The energy spent on removing an electron from an atom is called ionization energy and is designated Eo. Unless otherwise stated, the ionization energy is the energy of removal of the first electron, and there are also ionization energies for each subsequent electron.
Ionization energy of Cs: Eo = 376 kJ/mol
Cesium crystal lattice:
| 500 | Crystal cell | |
| 511 | Crystal grid #1 | |
| 512 | Lattice structure | Cubic body-centered |
| 513 | Lattice parameters | 6.140 Å |
| 514 | c/a ratio | |
| 515 | Debye temperature | 39.2 K |
| 516 | Name of space symmetry group | Im_ 3m |
| 517 | Symmetry space group number | 229 |
Production
Finding in nature is difficult due to the “dispersion” of cesium ores.
Canada is the leader in the production of cesium ore. The Bernick Lake deposit contains 70% of the world's reserves of the main cesium ore, pollucite. In Russia, deposits of cesium minerals have been discovered on the Kola Peninsula, in the Sayan Mountains and Transbaikalia.
Extraction of cesium from ore
Metal production is hampered by the difficulty of extracting highly purified metal from ores.
Cesium crystals
Production methods involve rectification, purification from solid impurities, removal of traces of gases (O2, H2, N2), and stepwise crystallization.
Interesting: cesium is stored in sealed containers filled with an inert gas, liquid or in a vacuum.
Alloys
The composition of alloys and compounds usually includes: barium, antimony, thallium. These alloys are produced by electrolysis.
| Alloy composition, alloy formula | Application |
| Cs3Sb (antimony) | Photocathodes |
| CsBi (bismuth) | Photocells |
| CsCl (chlorine) | In radiography, in the production of electrically conductive glass |
| CsI | In infrared optics, in particle detectors |
| CsBr | Included in phosphors |
Informative: the composition of the surface of Mars was carried out using a gamma spectrometer based on CsI (Tl).
Cesium compounds
Cesium forms binary compounds with most nonmetals. Cesium hydrides and deuterides are highly flammable in air, as well as in fluorine and chlorine atmospheres. Cesium compounds with nitrogen, boron, silicon and germanium are unstable and sometimes flammable and explosive. The halides and salts of most acids are more stable.
Connections with oxygen. Cesium forms nine compounds with oxygen, ranging in composition from Cs7O to CsO3.
Cesium oxide Cs2O forms brown-red crystals that diffuse in air. It is obtained by slow oxidation with an insufficient (2/3 of the stoichiometric) amount of oxygen. The remainder of unreacted cesium is distilled off in a vacuum at 180–200° C. Cesium oxide sublimes in a vacuum at 350–450° C, and decomposes at 500° C:
2Cs2O = Cs2O2 + 2Cs
Reacts vigorously with water to produce cesium hydroxide.
Cesium oxide is a component of complex photocathodes, special glasses and catalysts. It has been established that when producing synthol (synthetic oil) from water gas and styrene from ethylbenzene, as well as in some other syntheses, adding a small amount of cesium oxide (instead of potassium oxide) to the catalyst increases the yield of the final product and improves the process conditions.
Hygroscopic pale yellow crystals of cesium peroxide Cs2O2 can be obtained by oxidizing cesium (or its solution in liquid ammonia) with a dosed amount of oxygen. Above 650° C, cesium peroxide decomposes with the release of atomic oxygen and vigorously oxidizes nickel, silver, platinum and gold. Cesium peroxide dissolves in ice water without decomposition, and above 25 ° C it reacts with it:
2Cs2O2 + 2H2O = 4CsOH + O2
It dissolves in acids to form hydrogen peroxide.
When cesium is burned in air or oxygen, golden-brown cesium superoxide CsO2 is formed. Above 350°C it dissociates with the release of oxygen. Cesium superoxide is a very strong oxidizing agent.
Cesium peroxide and superoxide serve as sources of oxygen and are used for its regeneration in spacecraft and underwater vehicles.
Sesquioxide "Cs2O3" is formed in the form of a dark paramagnetic powder during the careful thermal decomposition of cesium superoxide. It can also be produced by oxidation of a metal dissolved in liquid ammonia or by controlled oxidation of peroxide. It is believed to be a dinadperoxide-peroxide [(Cs+)4(O22–)(O2–)2].
The orange-red ozonide CsO3 can be obtained by the action of ozone on anhydrous powder of cesium hydroxide or peroxide at low temperature. When standing, ozonide slowly decomposes into oxygen and superoxide, and upon hydrolysis it immediately turns into hydroxide.
Cesium also forms suboxides, in which the formal oxidation state of the element is significantly lower than +1. The oxide of composition Cs7O has a bronze color, its melting point is 4.3 ° C, and actively reacts with oxygen and water. In the latter case, cesium hydroxide is formed. When heated slowly, Cs7O decomposes into Cs3O and cesium. Violet crystals of Cs11O3 melt with decomposition at 52.5° C. Red-violet Cs4O decomposes above 10.5° C. The non-stoichiometric phase Cs2+xO changes composition up to Cs3O, which decomposes at 166° C.
Cesium hydroxide CsOH forms colorless crystals that melt at ° C. The melting temperatures of hydrates are even lower, for example, CsOH H2O monohydrate melts with decomposition at 2.5 ° C, and CsOH 3H2O trihydrate even -5.5 ° C.
Cesium hydroxide serves as a catalyst for the synthesis of formic acid. With this catalyst, the reaction occurs at 300° C without high pressure. The yield of the final product is very high - 91.5%.
Cesium halides CsF, CsCl, CsBr, CsI (colorless crystals) melt without decomposition and are volatile above the melting point. Thermal stability decreases when moving from fluoride to iodide; bromide and iodide in vapor partially decompose into simple substances. Cesium halides are highly soluble in water. In 100 g of water at 25 ° C, 530 g of cesium fluoride, 191.8 g of cesium chloride, 123.5 g of cesium bromide, 85.6 g of cesium iodide are dissolved. Anhydrous chloride, bromide and iodide crystallize from aqueous solutions. Cesium fluoride is released in the form of crystalline hydrates of the composition CsF nH2O, where n = 1, 1.5, 3.
When interacting with halides of many elements, cesium halides easily form complex compounds. Some of them, for example Cs3[Sb2Cl6], are used for the isolation and analytical determination of cesium.
Cesium fluoride is used to produce organofluorine compounds, piezoelectric ceramics, and special glasses. Cesium chloride is an electrolyte in fuel cells, a flux for welding molybdenum.
Cesium bromide and iodide are widely used in optics and electrical engineering. The crystals of these compounds are transparent to infrared rays with wavelengths from 15 to 30 μm (CsBr) and from 24 to 54 μm (CsI). Conventional prisms made of sodium chloride transmit rays with a wavelength of 14 microns, and those of potassium chloride - 25 microns, so the use of cesium bromide and iodide instead of sodium and potassium chlorides made it possible to record the spectra of complex molecules in the far infrared region.
If, when making fluorescent screens for televisions and scientific equipment, approximately 20% cesium iodide is introduced between zinc sulfide crystals, the screens will absorb X-rays better and glow brighter when irradiated with an electron beam.
Scintillation instruments for detecting heavy charged particles containing single crystals of cesium iodide activated by thallium have the highest sensitivity of all instruments of this type.
Advantages and disadvantages
The advantages of the metal include its incredible activity. It has no equal in sensitivity to light. The easiest way to obtain plasma from cesium is energy-efficiently.
Interesting: many physicists believe that it is advisable to create plasma using the energy of atomic reactors. This will make it possible to directly convert thermal energy into electrical energy.
Disadvantages include the difficulty of working with metal and the limited supply of cesium-containing ores.
Informative: the 137Cs isotope is a source of high radioactivity in the Chernobyl area.
Uses of cesium
The metal is used in engines of orbiting satellites and in MHD generators.
Application:
- In current sources for fuel cells.
- In atomic clocks (the error is 1 second per 100 million years). The transition frequency for 137Cs is 9193 MHz, called the "cesium standard". This is the main time standard in the transmission of GPS, Internet, and mobile data.
- Radioactive 137Cs is used to treat cancer.
- In optical instruments (weapon sights, binoculars, noctovisors).
- Lasers with very high efficiency.
Educational: Japanese scientists have invented a fabric that can absorb radioactive cesium from water and soil.
Structure
Regardless of activity, all metals have a common structure. Atoms in a simple metal are not arranged chaotically, as in amorphous substances, but in an orderly manner - in the form of a crystal lattice. A metal bond holds the atoms in one position.
This type of connection is carried out due to positively charged ions located at the nodes of a crystal cell (lattice unit), and negatively charged free electrons, which form the so-called electron gas. Electrons separated from atoms, turning them into ions, and began to move randomly in the lattice, holding the ions together. Without electrons, the lattice would disintegrate due to the rejection of equally charged ions.
There are three types of crystal lattice. Body-centered cubic consists of 9 ions and is characteristic of chromium, iron, and tungsten. Face-centered cubic contains 14 ions and is characteristic of lead, aluminum, and silver. A hexagonal close-packed lattice of zinc, titanium, and magnesium consists of 17 ions.
Rice. 2. Types of crystal lattices.
Melting point table for medium-melting metals and alloys:
| Metal name | Melting point, oC |
| Antimony | 630,63 |
| Neptunium | 639 |
| Plutonium | 639,4 |
| Magnesium | 650 |
| Aluminum | 660,32 |
| Radium | 700 |
| Barium | 727 |
| Strontium | 777 |
| Cerium | 795 |
| Ytterbium | 824 |
| Europium | 826 |
| Calcium | 841,85 |
| Lanthanum | 920 |
| Praseodymium | 935 |
| Germanium | 938,25 |
| Silver | 961,78 |
| Neodymium | 1024 |
| Promethium | 1042 |
| Actinium | 1050 |
| Gold | 1064,18 |
| Samarium | 1072 |
| Copper | 1084,62 |
| Uranus | 1132,2 |
| Manganese | 1246 |
| Beryllium | 1287 |
| Gadolinium | 1312 |
| Terbium | 1356 |
| Dysprosium | 1407 |
| Nickel | 1455 |
| Holmium | 1461 |
| Cobalt | 1495 |
| Yttrium | 1526 |
| Erbium | 1529 |
| Iron | 1538 |
| Scandium | 1541 |
| Thulium | 1545 |
| Palladium | 1554,9 |
| Protactinium | 1568 |
Properties
The structure of the crystal lattice determines the basic physical and chemical properties of metals. Metals shine, melt, and conduct heat and electricity. Industry and metallurgy have found application for the physical properties of metals in the manufacture of parts, foil, machine bodies, mirrors, household and industrial chemicals. The characteristics of metals and their uses are presented in the table of physical properties of metals.
| Properties | Peculiarities | Examples | Application |
| Metallic shine | Ability to reflect sunlight | The most shiny metals are Hg, Ag, Pd | Making mirrors |
| Density | Light - have a density of less than 5 g/cm3 | Na, K, Ba, Mg, Al. The lightest metal is lithium with a density of 0.533 g/cm3 | Manufacturing of cladding and aircraft parts |
| Heavy - have a density greater than 5 g/cm3 | Sn, Fe, Zn, Au, Pb, Hg. The heaviest is osmium with a density of 22.5 g/cm3 | Use in alloys | |
| Plastic | Ability to change shape without destruction (can be rolled into thin foil) | The most ductile are Au, Cu, Ag. Brittle – Zn, Sn, Bi, Mn | Forming, pipe bending, wire making |
| Hardness | Soft - cut with a knife | Na, K, In | Making soap, glass, fertilizers |
| Hard – comparable in hardness to diamond | The hardest is chrome, it cuts glass. | Manufacturing of load-bearing structures | |
| Melting temperature | Low-melting - melting point below 1000°C | Hg (38.9°C), Ga (29.78°C), Cs (28.5°C), Zn (419.5°C) | Production of radio equipment, tinplate |
| Refractory – melting point above 1000°C | Cr (1890°С), Mo (2620°С), V (1900°С). The most refractory is tungsten (3420°C) | Manufacturing of incandescent lamps | |
| Thermal conductivity | Ability to transfer heat to other bodies | Ag, Cu, Au, Al conduct current and heat best | Cooking in metal containers |
| Electrical conductivity | The ability to conduct electric current due to free electrons | Transmission of electricity through wires |
Rice. 3. Examples of the use of metals.
Melting point table for low-melting metals and alloys:
| Metal name | Melting point, oC |
| Mercury | -38,83 |
| France | 25 |
| Cesium | 28,44 |
| Gallium | 29,7646 |
| Rubidium | 39,3 |
| Potassium | 63,5 |
| Sodium | 97,81 |
| Indium | 156,5985 |
| Lithium | 180,54 |
| Tin | 231,93 |
| Polonium | 254 |
| Bismuth | 271,3 |
| Thallium | 304 |
| Cadmium | 321,07 |
| Lead | 327,46 |
| Zinc | 419,53 |
What have we learned?
From the 9th grade lesson we learned about the physical properties of metals. We briefly examined the position of metals in the periodic table and the structural features of the crystal lattice. Due to their structure, metals have plasticity, hardness, the ability to melt, and conduct electric current and heat. The properties of metals are heterogeneous. There are light and heavy metals, fusible and refractory, soft and hard. Physical properties are used to make alloys, electrical wires, dishes, soap, glass, and structures of various shapes.
Melting point table for refractory metals and alloys:
| Metal name | Melting point, oC |
| Lutetium | 1652 |
| Titanium | 1668 |
| Thorium | 1750 |
| Platinum | 1768,3 |
| Zirconium | 1855 |
| Chromium | 1907 |
| Vanadium | 1910 |
| Rhodium | 1964 |
| Technetium | 2157 |
| Hafnium | 2233 |
| Ruthenium | 2334 |
| Iridium | 2466 |
| Niobium | 2477 |
| Molybdenum | 2623 |
| Tantalum | 3017 |
| Osmium | 3033 |
| Rhenium | 3186 |
| Tungsten | 3422 |