How to identify titanium and distinguish it from other metals?
Identification of certain metals is an accurate and simple process only if you have special laboratory equipment, a spectrometer in particular. At home, the task becomes much more complicated. It is especially difficult to distinguish between materials that are similar in color and magnetic properties. However, even in such a situation, there are practical methods to distinguish titanium from other metals. The most interesting for comparison are aluminum and steel, including stainless steel. Here, even experienced craftsmen who regularly work with metals and accept titanium scrap are not always able to clearly identify what exactly they have in their hands.
The dual properties of titanium metal
Many people are interested in the slightly mysterious and not fully studied titanium - a metal whose properties are somewhat ambiguous. Metal is both the strongest and most fragile.
It was discovered by two scientists with a difference of 6 years - the Englishman W. Gregor and the German M. Klaproth. The name titan is associated, on the one hand, with the mythical titans, supernatural and fearless, and on the other hand, with Titania, the queen of fairies. This is one of the most common materials in nature, but the process of obtaining pure metal is particularly complex.
How to distinguish titanium from steel, aluminum
The first pair is non-ferrous and ferrous metals. Most steels have magnetic properties. The exception is alloyed metals of the austenitic class. A striking example is stainless steel with a high nickel content. This grade of steel, like titanium, is paramagnetic. Therefore, the standard option using a magnet is unacceptable here.
There are three reliable ways to determine titanium at home :
- mathematical;
- graphic;
- abrasive;
- galvanic.
The designations are quite conventional; below we will cover each of the options in detail.
Stainless steels: how composition affects properties
Alloy steels occupy a significant share of the metallurgical products market. These include the so-called “stainless steels” - a group of alloys characterized by increased resistance to corrosion. Since their appearance, the range of such steels has expanded to several hundred items. Therefore, a system for their classification and labeling was developed.
It is worth noting that the name “stainless steel” does not entirely correctly reflect its properties. Any iron-carbon alloy is exposed to oxygen and aggressive substances, but for this to affect its performance properties, it takes different times. Therefore, stainless steels are more correctly called corrosion-resistant.
By composition
Chromium, nickel, vanadium, molybdenum, titanium and some others are used as alloying additives that increase the resistance of the iron-carbon alloy to rust formation. Corrosion resistance is also increased by manganese and silicon introduced to deoxidize and neutralize sulfur. Based on the main alloying elements, stainless steels are classified as chromium, manganese, etc. Some additives are used to impart special structural or technological properties to steels, for example, to crush carbides and increase impact strength.
The basic alloying elements of stainless steel are chromium and nickel. They both enter into solid solution with iron and increase corrosion resistance. When oxidized, they form a thin oxygen-impermeable film on the surface of a steel product, resistant to chemical, electrochemical and atmospheric influences. Nickel expands the austenite region in iron-carbon alloys. Chromium narrows it, but is a carbide-forming element and binds carbon. The ratio of nickel and chromium has a decisive influence on impact strength, weldability and ability to withstand cold deformation.
Carbon, as one of the essential components of steels, negatively affects corrosion resistance. However, the hardness and wear resistance of steel depends on its content. For example, 95Х18 has less pronounced corrosion-resistant properties compared to 40Х13, despite its higher chromium content.
By properties
A more clear idea of alloys is given by dividing them into groups according to properties:
- Corrosion resistant. Steels are highly resistant to atmospheric corrosion and can be used under normal conditions in a loaded state. Examples include stainless steel used for the manufacture of utensils and equipment for the food industry: 08Х18Н10, 20Х13, 30Х13.
- Heat resistant. A distinctive feature of such alloys is their high resistance to scale formation at high temperatures. Heat-resistant stainless steels are used for the manufacture of heat exchangers for boiler and pyrolysis plants (15Х28), valves for automobile and aircraft engines (40Х10С2М), parts for heating metallurgical furnaces (10Х23Н18).
- Heat resistant . A number of alloys have been developed that can operate under load at high temperatures without significant deformation and destruction. They use complex alloying systems (05Х27У5, 15Х12ВН14Ф, 37Х12Н8Г8МФБ). Steel type 20Х13 also has moderate heat resistance.
By structure
Based on their microstructure, stainless steels are divided into the following classes:
- austenitic;
- ferritic;
- martensitic;
In addition to them, there are intermediate groups:
- austenitic-ferritic;
- martensitic-ferritic;
- martensitic-carbide.
Heat treatment has a great influence on corrosion resistance, since it affects the phase composition of most stainless steels. Stability decreases when carbide heterogeneity occurs. This phenomenon is caused by the so-called intercrystalline corrosion. When steels are heated to temperatures in the range of 500 – 800 °C, chains of carbides and areas with a reduced chromium content are formed at the grain boundaries. In the body of the grain, the content of alloying elements remains high. This type of corrosion is often observed in weld areas. To combat this phenomenon, the steel composition is stabilized by introducing a small amount of titanium.
Austenitic steels
During crystallization, austenitic steels form a single-phase system with a face-centered crystal lattice. One of the most prominent representatives of the class is alloy 08Х18Н10. Due to the high nickel content in stainless steels of this class (up to 30%), the austenite phase remains stable down to – 200 °C, and the carbon content does not exceed 0.12%. Steels with this structure are characterized by the absence of magnetic properties. Most of them have good machinability.
Austenitic steels are necessarily subjected to heat treatment - hardening, tempering or annealing. The cooling rate practically does not change the hardness, but it affects the resistance to liquid and gaseous aggressive media, stabilizes the grain size and resistance to deformation.
Additional elements are introduced into the alloying systems of austenitic chromium-nickel steels:
- molybdenum – to prevent pitting and operation in reducing atmospheres
- titanium and niobium – for protection against intercrystalline corrosion.
- silicon – to increase acid resistance;
- manganese - to improve casting qualities.
Pure mathematics
In this approach, metal identification is done by weight. The disadvantage of the method occurs when only one type of metal is available . To determine in your hands that it cannot be made heavier, you have to resort to mathematical calculations. This is facilitated by significant differences in the density of metals:
- titanium – 4.5;
- iron – 7.8;
- aluminum and duralumin – 2.7.
To use this method for determining titanium in your household, you need to have accurate scales.
Parameter values are given in g/cc. It remains to add that the density of steel depends on the specific grade of metal. However, in absolute terms these differences are insignificant. Therefore, the density of steel can be safely taken as the value of a similar characteristic of iron.
All that remains is to clarify the volume and weight of the part or piece of metal. Further, simple calculations will show whether it is aluminum, steel or the desired metal - titanium. How to determine the volume of a part with a complex shape? The best option here is Archimedes' law. The mass of the ejected liquid, when immersing a metal structure, allows you to determine its volume. The situation is simplified by the density of water, equivalent to 1 kg/cubic dm. Accordingly, each gram of ejected liquid is equal to one cubic centimeter of volume.
Of course, this is a tedious, complex and inaccurate method, but in order to determine titanium at home, it has its place.
This is what titanium metal looks like
Areas of use
The use of titanium depends on the degree of its purification from impurities. The presence of even a small amount of other chemical elements in the composition of a titanium alloy radically changes its physical and mechanical characteristics.
Titanium with a certain amount of impurities is called technical titanium. It has high corrosion resistance, it is a lightweight and very durable material. Its use depends on these and other indicators.
- In the chemical industry, heat exchangers, pipes of various diameters, fittings, housings and parts for pumps for various purposes are made from titanium and its alloys. The substance is indispensable in places where high strength and resistance to acids are required.
- In transport, titanium is used for the manufacture of parts and assemblies for bicycles, cars, railway cars and trains. The use of the material reduces the weight of rolling stock and cars, and gives lightness and strength to bicycle parts.
- Titanium is of great importance in the naval department. Parts and hull elements for submarines, propellers for boats and helicopters are made from it.
- The construction industry uses zinc-titanium alloy. It is used as a finishing material for facades and roofs. This very durable alloy has an important property: it can be used to make architectural parts of the most fantastic configuration. It can take any form.
- In the last decade, titanium has been widely used in the oil industry. Its alloys are used in the manufacture of equipment for ultra-deep drilling. The material is used to manufacture equipment for offshore oil and gas production.
Pure titanium has its own areas of application. It is needed where resistance to high temperatures is required while maintaining the strength of the metal.
It is used in:
- aircraft manufacturing and space industry for the manufacture of skin parts, housings, fastening elements, landing gear;
- medicine for prosthetics and the manufacture of heart valves and other devices;
- technology for working in the cryogenic area (here the property of titanium is used - as the temperature decreases, the strength of the metal increases and its ductility is not lost).
In percentage terms, the use of titanium for the production of various materials looks like this:
- 60% is used for paint production;
- plastic consumes 20%;
- 13% are used in paper production;
- mechanical engineering consumes 7% of the produced titanium and its alloys.
The raw materials and process for producing titanium are expensive, the costs of its production are compensated and paid off by the service life of products made from this substance, its ability not to change its appearance over the entire period of operation.
Drawings on glass
This is the most accessible method of how to distinguish titanium at home, but you need to master it and have experience working with titanium. Metal leaves characteristic indelible marks on glass and tiles . It is enough to run the sharpened edge of the metal along one of the specified materials. These are marks, not scratches. Windows of public transport are often painted in a similar way. You can wash titanium graphics on tiles with a solution of hydrofluoric acid; you should handle it with extreme caution.
This method is simple and effective. Titanium, contrary to popular belief, leaves a mark even on dirty glass. So there is no need to degrease its surface. On the contrary, any grade of steel and aluminum can barely scratch the glass. This is an excellent method to identify titanium.
Basics of protecting titanium from corrosion development
There are several most common means that can greatly reduce the risk of removing the protective film on the surface of the material.
There are several most common methods:
- Rationalization of the structure of the structure. It is necessary to pay attention to where exactly the part is used, whether there is a potentially dangerous neighborhood that can stimulate the appearance of the process of electrochemical corrosion. It is best that the structure of the product is such that it can be quickly and easily cleaned of accumulated dirt and various potentially hazardous substances.
- Working with the environment. You need to pay attention to whether the environment in which the titanium product is used is dangerous. It is possible to influence the environment using different types of additives. This way, solutions of acids and alkalis can be made less aggressive and the duration of use can be increased without potential external problems.
- Application of a special protective coating to the material. The main thing that such a coating provides is the prevention of metal contact with aggressive environments and oxidation catalysts. It is necessary to pay attention to ensure that such coating maintains its uniformity and integrity throughout the entire period of operation. If necessary, this coating can be further updated.
Our company provides services for high-quality protection of materials from corrosion.
We are ready to answer all customer questions, as well as quickly prepare everything needed to eliminate potential oxidation risks when using titanium products. Return to articles Share article
Abrasive wheel
An ideal way to distinguish titanium from stainless steel for owners of a sharpening machine (which, in fact, is not at all necessary). However, almost any abrasive surface will do, even asphalt. The contact of titanium with an abrasive is accompanied by a scattering of rich white sparks. The interaction of steel with an abrasive surface is characterized by a yellow or red tint. There are significantly fewer sparks.
Stainless steel grades are fireproof. Processing of certain brands of stainless steel occurs without sparks at all. This property is used in fire hazardous industries. Only stainless steel tools are allowed there. A similar technique is used in the question of how to distinguish titanium from aluminum. Grinding of the latter on an abrasive wheel also occurs practically without sparks.
This method of determining titanium can be called the most effective - the color of the spark will indeed be different from other metals. In general, the spark test is one of the most popular and correct for determining and recognizing different metals.
Video - how to distinguish titanium from magnesium and aluminum:
Other techniques
There are a number of alternative ways to determine titanium in your hands or aluminum, for example. One option is thin shavings. In the case of titanium, it is highly flammable and burns brightly. On the contrary, aluminum shavings melt. When duralumin “metal filings” are placed in an alkaline solution, active evolution of hydrogen is observed.
The next way to distinguish titanium metal from steel and aluminum is thermal conductivity. The numerical values of the parameter W/(m K) for the indicated metals are:
- titanium – 14;
- low carbon steel – 55;
- stainless steel – 16;
- aluminum – 250.
Titanium products are warmer in the hands. Of course, the approach is not characterized by high accuracy, and is generally unsuitable .
Summary
As you can see, even at home, it is quite possible to distinguish titanium from aluminum and steel. The most practical options are spark and glass . For the first case, any abrasive surface is sufficient, even asphalt or hardened concrete. The bright sparkle of titanium is successfully used by bikers by installing horseshoes made of this metal on their shoes. The mark on the glass is beneficial because the metal is not damaged. A relative disadvantage is that some titanium alloys do not leave a pattern. But for pure metal this is the best option.
Features of titanium and its alloys
Today, titanium ranks 4th in terms of industrial use. However, its active extraction and production begins only in the 40s of the 20th century. Titanium and its alloys have unique characteristics and require more careful consideration in metalworking.
Titanium
Basic information
Titanium is a silver-colored metal that is included in group 4 of period 4 in the periodic table. According to official data, it ranks 10th in distribution in nature.
Initially, the metal was used in the national economy, but after its super strength and low specific gravity were discovered, titanium and its alloys began to be used in the construction of aircraft, ships, rockets and cars.
History of discovery
Titanium oxide was first discovered in 1791. This discovery was made by W. Gregor (English). He took a sample of ferruginous sand on a Cornwall beach and carried out research on it. As a result of experiments, the scientist isolated an oxide of an unknown metal, which he never gave a name. Another scientist called this element titanium - Martin Heinrich Klaproth (German). In 1825, another researcher, Jöns Jakob Berzelius, was able to isolate a sample of this metal from its oxide.
Production and manufacturing
Due to its prevalence in nature, mining ore containing titanium is not difficult. The most common types of ore that contain this metal are brookite, ilmenite, anatase and rutile. However, further processing of titanium (melting, hardening and aging) is considered expensive. There are several stages in obtaining pure metal from ore:
- First of all, titanium slag is extracted by heating ilmenite to 1650 degrees.
- Next, the slag goes through a chlorination process.
- After this, titanium sponge is produced using resistance furnaces.
- To obtain pure metal, the final processing step is the refining process.
If you need to obtain titanium ingots, a sponge based on it is melted in a vacuum furnace.
Advantages and disadvantages
Like any other metal, titanium has its strengths and weaknesses. Benefits include:
- light weight;
- corrosion resistance;
- resistance to high temperatures;
- high strength - greater than that of the best steels.
Flaws:
- Dust and chips remaining after processing titanium workpieces can ignite at a temperature of 400 degrees.
- This metal is difficult to weld and is practically impossible to cut.
- The costly method of obtaining metal from ore causes its high cost.
However, despite the existing disadvantages, the material and its alloys are widely used in various industries. Light weight
Titanium Products
In construction stores you can find a variety of products made from this metal. It is used to produce wire, tape and foil, rods, and pipes. You can also purchase titanium in solid sheets.
Application area
Due to the advantages that titanium has, it is used in various industries:
- naval affairs;
- construction;
- medicine;
- mechanical engineering;
- shipbuilding and aircraft manufacturing;
- chemical industry.
The peculiarities of the use of this metal make it more popular every year. It is actively used in the national economy.
Characteristics and properties
The characteristics of titanium directly depend on the amount of impurities contained in its composition. Physical parameters:
- Specific strength - 450 MPa.
- Melting point - 1668 degrees.
- Boiling point - 3227 degrees.
- The tensile strength of the alloys is 2000 MPa.
- The elasticity of titanium is 110.25 GPa.
- Metal hardness - 103 HB.
- The yield strength is 380 MPa.
Use of abrasives
When processing metal on a grinding machine or during sharp longitudinal friction on the abrasive surface of a grindstone, the contact of titanium is accompanied by a scattering of bright white sparks. If there is no abrasive, you can use a fine file or even plain concrete, although the effect will be less.
Sparks from stainless steel have yellow and red tints. Much less of them fly out, and on concrete and files there will be none at all. Some grades of stainless steel have been developed to be fire resistant. Sparking during processing of such metals is not technologically possible. When aluminum rubs against a forming surface, sparks are not released, but characteristic silvery marks may remain on the surface.
This test for the possibility of spark formation is the most popular and simplest, since the color really differs very much, and their complete absence immediately indicates that this metal is not titanium.
Galvanic reaction test
To perform this test, you will need a DC source with a voltage of about 12 V. This can be a car battery or a converter transformer. Connect the plus wire of the battery to the test sample, and the minus wire to a metal rod, at the end of which cotton wool, gauze or a piece of cotton fabric is wound. Wet cotton wool with a weak solution of hydrochloric acid or regular Coca-Cola.
If it is titanium, then when you touch the metal, its surface will become colored as a result of the formation of an oxide film. The color shade depends on the voltage, the concentration of the acid in the solution and the exposure time. Stainless alloys and aluminum are not susceptible to this reaction.
Specific gravity comparison
Everyone knows that aluminum is the lightest of these three metals, and steel is the heaviest. But how can you determine if you have one sample and nothing to compare with? This can be done by measuring and calculating the density or specific gravity of the material, which is approximately:
- 2.7 g/cm3 for aluminum;
- 4.5 g/cm3 for titanium;
- 7.8 g/cm3 for stainless steel.
This method of determination requires precise scales and a container for immersing the sample in water.
After weighing the metal, it is necessary to determine its volume. The easiest way to do this is to use Archimedes’ law, known from school, by immersing the sample in a liquid. A change in water level will show the desired value.
This is a more complex and lengthy definition option and is therefore used very rarely. But it also produces results and should be considered.
About other properties of titanium
In some cases, metal determination can be done in simple and very original ways:
- titanium shavings ignite and burn quite easily;
- this metal is a good heat insulator and when one edge of the sample is heated, the rest of the sample will be cold;
- low thermal conductivity gives the feeling of a warm object in the hands, unlike cold steel and aluminum.
And lastly, hit the sample with a hammer, as a result there will be no marks on the steel, a small dent will form on the titanium, and the aluminum will suffer the most.
How to identify real titanium?
In nature, titanium is found in minerals such as titanite, ilmenite or rutile. In its pure form it is a silver-gray light metal with the highest specific strength. In appearance it is difficult to distinguish it from aluminum or stainless steel. The magnet method will not work here, since magnets also do not attract aluminum or most stainless steels. To dispel all doubts that you have a sample of real titanium in your hands, you need to conduct one of the tests available to you.
Titanium
Titanium Basics
Titanium
is a chemical element with atomic number 22, atomic weight 47.88, a light silvery-white metal. Density 4.51 g/cm3, Tmelt=1668+(-)5 °C, Tboil=3260 °C. Titanium and titanium alloys combine lightness, strength, high corrosion resistance, low coefficient of thermal expansion, and the ability to operate in a wide temperature range.
History of the discovery of titanium
Titanium oxide TiO2 was first discovered in 1789 by the English scientist, specialist in the field of mineralogy W. Gregor, who, while studying magnetic ferrous sand, isolated an oxide of an unknown metal, calling it menakenova. The first sample of metallic titanium was obtained in 1825 by the Swedish chemist and mineralogist J. Ya. Berzelius.
Properties of titanium
In the periodic table of elements of D.I. Mendeleev, titanium is located in group IV of the 4th period at number 22. In the most important and most stable compounds, the metal is tetravalent. It looks like steel. Titanium is a transition element. This metal melts at a fairly high temperature (1668±4 °C) and boils at 3300 °C, the latent heat of melting and evaporation of titanium is almost twice as high as that of iron.
There are two known allotropic modifications of titanium (two varieties of titanium that have the same chemical composition, but different structure and properties). Low-temperature alpha modification, existing up to 882.5 °C and high-temperature beta modification, stable from 882.5 °C to the melting point.
In terms of density and specific heat capacity, titanium occupies an intermediate position between the two main structural metals: aluminum and iron. It is also worth noting that its mechanical strength is approximately twice that of pure iron and almost six times that of aluminum. But titanium can actively absorb oxygen, nitrogen and hydrogen, which sharply reduce the plastic properties of the metal. With carbon, titanium forms refractory carbides with high hardness.
Titanium has low thermal conductivity, which is 13 times less than the thermal conductivity of aluminum and 4 times less than that of iron. The coefficient of thermal expansion at room temperature is relatively small; it increases with increasing temperature.
The elastic moduli of titanium are low and exhibit significant anisotropy. Elastic moduli characterize the ability of a material to elastically deform when a force is applied to it. Anisotropy is the difference in elasticity properties depending on the direction of the force. With an increase in temperature to 350 °C, the elastic moduli decrease almost linearly. The small value of the elastic modulus of titanium is its significant drawback, because in some cases, in order to obtain sufficiently rigid structures, it is necessary to use larger sections of products compared to those that follow from the strength conditions.
Titanium has a fairly high electrical resistivity, which, depending on the impurity content, ranges from 42·10-8 to 80·10-6 Ohm·cm. At temperatures below 0.45 K it becomes a superconductor.
Titanium is a paramagnetic metal. Typically, for paramagnetic substances, the magnetic susceptibility decreases when heated. Magnetic susceptibility characterizes the relationship between the magnetization of a substance and the magnetic field in this substance. Titanium is an exception to this rule - its susceptibility increases significantly with temperature.
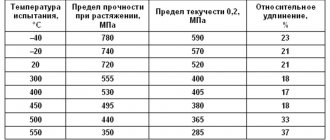
Characteristics of physical and mechanical properties of titanium (VT1-00)
| Density r, kg/m3 | 4,5 × 10–3 |
| Melting temperature T pl , ° C | 1668± 4 |
| Linear expansion coefficient a × 10–6, deg–1 | 8,9 |
| Thermal conductivity l, W/(m × deg) | 16,76 |
| Tensile strength s in, MPa | 300–450 |
| Conditional yield strength s 0.2 , MPa | 250–380 |
| Specific strength (s in /r × g )× 10–3, km | 7–10 |
| Relative elongation d,% | 25–30 |
| Relative narrowing Y, % | 50–60 |
| Modulus of normal elasticity E´ 10–3, MPa | 110,25 |
| Shear modulus G´ 10–3, MPa | 41 |
| Poisson's ratio m, | 0,32 |
| Hardness HB | 103 |
| Impact strength KCU, J/cm2 | 120 |
Titanium has two polymorphic modifications: a-titanium with a hexagonal close-packed lattice with periods a
= 0.296 nm,
c
= 0.472 nm and a high-temperature modification of b-titanium with a cubic body-centered lattice with a period of
a
= 0.332 nm at 900 ° C. The temperature of the polymorphic a « b - transformation is 882 ° C.
The mechanical properties of titanium significantly depend on the impurity content in the metal. There are interstitial impurities - oxygen, nitrogen, carbon, hydrogen and substitutional impurities, which include iron and silicon. Although impurities increase strength, they at the same time sharply reduce ductility, and interstitial impurities, especially gases, have the strongest negative effect. With the introduction of only 0.003% H, 0.02% N or 0.7% O, titanium completely loses the ability to undergo plastic deformation and breaks brittlely.
Hydrogen is especially harmful, causing hydrogen embrittlement
titanium alloys. Hydrogen enters the metal during melting and subsequent processing, in particular during pickling of semi-finished products. Hydrogen is slightly soluble in a-titanium and forms plate-like hydride particles, which reduce impact strength and are especially negative in delayed fracture tests.
Therefore, the content of impurities, especially gases, in titanium and titanium alloys (Tables 17.1, 17.2) is strictly limited.
The industrial method of titanium production consists of enrichment and chlorination of titanium ore, followed by its reduction from titanium tetrachloride with magnesium metal (magnesium-thermal method). Titanium sponge obtained by this method
(GOST 17746–79), depending on the chemical composition and mechanical properties, the following grades are produced: TG-90, TG-100, TG-110, TG-120, TG-130, TG-150, TG-TV (see table. 17.1). The numbers mean Brinell hardness HB, TV - hard.
To obtain monolithic titanium, the sponge is ground into powder, pressed and sintered, or melted in arc furnaces in a vacuum or an atmosphere of inert gases.
The mechanical properties of titanium are characterized by a good combination of strength and ductility. For example, technically pure titanium grade VT1-0 has: s in = 375–540 MPa, s 0.2 = 295–410 MPa, d ³
20%, and in terms of these characteristics is not inferior to a number of carbon and Cr-Ni corrosion-resistant steels.
The high plasticity of titanium compared to other metals with an hcp lattice (Zn, Mg, Cd) is explained by a large number of slip and twinning systems due to the low ratio with
/
a
= 1.587. Apparently, this is related to the high cold resistance of titanium and its alloys (for more details, see Chapter 13).
When the temperature rises to 250 ° C, the strength of titanium decreases almost 2 times. However, heat-resistant Ti alloys have no equal in terms of specific strength in the temperature range of 300–600 ° C; at temperatures above 600 ° C, titanium alloys are inferior to alloys based on iron and nickel.
Titanium has a low normal elastic modulus ( E
= 110.25 GPa) - almost 2 times less than that of iron and nickel, which makes it difficult to manufacture rigid structures.
Titanium is one of the chemically active metals, but it has high corrosion resistance, since a stable passive film of TiO2 is formed on its surface, firmly bonded to the base metal and excluding its direct contact with the corrosive environment. The thickness of this film usually reaches 5–6 nm.
Thanks to the oxide film, titanium and its alloys do not corrode in the atmosphere, in fresh and sea water, and are resistant to cavitation corrosion and stress corrosion, as well as acids of organic origin.
The production of products from titanium and its alloys has a number of technological features. Due to the high chemical activity of molten titanium, its melting, casting and arc welding are carried out in a vacuum or in an atmosphere of inert gases.
During process and operational heating, especially above 550–600 ° C, it is necessary to take measures to protect titanium from oxidation and gas saturation (alpha layer) (see Chapter 3).
Titanium can be pressed well when hot and satisfactorily when cold. It is easily rolled, forged, and stamped. Titanium and its alloys are well welded by resistance and argon arc welding, providing high strength and ductility of the welded joint. The disadvantage of titanium is its poor machinability due to its tendency to stick, low thermal conductivity and poor anti-friction properties.
The main purpose of alloying titanium alloys is to increase strength, heat resistance and corrosion resistance. Alloys of titanium with aluminum, chromium, molybdenum, vanadium, manganese, tin and other elements are widely used. Alloying elements have a great influence on the polymorphic transformations of titanium.
Table 17.1
Grades, chemical composition (%) and hardness of titanium sponge (GOST 17746–79)
| Brand | Ti, no less | No more | Hardness NV, 10/1500/30, no more | ||||||
| Fe | Si | Ni | C | Cl | N | O | |||
| TG-90 | 99,74 | 0,05 | 0,01 | 0,04 | 0,02 | 0,08 | 0,02 | 0,04 | 90 |
| TG-100 | 99,72 | 0,06 | 0,01 | 0,04 | 0,03 | 0,08 | 0,02 | 0,04 | 100 |
| TG-110 | 99,67 | 0,09 | 0,02 | 0,04 | 0,03 | 0,08 | 0,02 | 0,05 | 110 |
| TG-120 | 99,64 | 0,11 | 0,02 | 0,04 | 0,03 | 0,08 | 0,02 | 0,06 | 120 |
| TG-130 | 99,56 | 0,13 | 0,03 | 0,04 | 0,03 | 0,10 | 0,03 | 0,08 | 130 |
| TG-150 | 99,45 | 0,2 | 0,03 | 0,04 | 0,03 | 0,12 | 0,03 | 0,10 | 150 |
| TG-TV | 99,75 | 1,9 | – | – | 0,10 | 0,15 | 0,10 | – | – |
Table 17.2
Grades and chemical composition (%) of wrought titanium alloys (GOST 19807–91)
| Brand designations | Ti | Al | V | Mo | Sn | Zr | Mn | Cr | Si | Fe | O | H | N | C |
| VT1-00 | The basis | – | – | – | – | – | – | – | 0,08 | 0,15 | 0,10 | 0,008 | 0,04 | 0,05 |
| VT1-0 | Same | – | – | – | – | – | – | – | 0,10 | 0,25 | 0,20 | 0,010 | 0,04 | 0,07 |
| VT1-2 | Same | – | – | – | – | – | – | – | 0,15 | 1,5 | 0,30 | 0,010 | 0,15 | 0,10 |
| OT4-0 | Same | 0,4–1,4 | – | – | – | 0,30 | 0,5–1,3 | – | 0,12 | 0,30 | 0,15 | 0,012 | 0,05 | 0,10 |
| OT4-1 | Same | 1,5–2,5 | – | – | – | 0,30 | 0,7–2,0 | – | 0,12 | 0,30 | 0,15 | 0,012 | 0,05 | 0,10 |
| OT4 | Same | 3,5–5,0 | – | – | – | 0,30 | 0,8–2,0 | – | 0,12 | 0,30 | 0,15 | 0,012 | 0,05 | 0,10 |
| VT5 | Same | 4,5–6,2 | 1,2 | 0,8 | – | 0,30 | – | – | 0,12 | 0,30 | 0,20 | 0,015 | 0,05 | 0,10 |
| VT5-1 | Same | 4,3–6,0 | 1,0 | – | 2,0 –3,0 | 0,30 | – | – | 0,12 | 0,30 | 0,15 | 0,015 | 0,05 | 0,10 |
| VT6 | Same | 5,3–6,8 | 3,5–5,3 | – | – | 0,30 | – | – | 0,10 | 0,60 | 0,20 | 0,015 | 0,05 | 0,10 |
| VT6s | Same | 5,3–6,5 | 3,5–4,5 | – | – | 0,30 | – | – | 0,15 | 0,25 | 0,15 | 0,015 | 0,04 | 0,10 |
| VT3-1 | Same | 5,5–7,0 | – | 2,0–3,0 | – | 0,50 | – | 0,8–2,0 | 0,15–0,40 | 0,2–0,7 | 0,15 | 0,015 | 0,05 | 0,10 |
| VT8 | Same | 5,8–7,0 | – | 2,8–3,8 | – | 0,50 | – | – | 0,20–0,40 | 0,30 | 0,15 | 0,015 | 0,05 | 0,10 |
| VT9 | Same | 5,8–7,0 | – | 2,8–3,8 | – | 1,0–2,0 | – | – | 0,20–0,35 | 0,25 | 0,15 | 0,015 | 0,05 | 0,10 |
| VT14 | Same | 3,5–6,3 | 0,9–1,9 | 2,5–3,8 | – | 0,30 | – | – | 0,15 | 0,25 | 0,15 | 0,015 | 0,05 | 0,10 |
| VT20 | Same | 5,5–7,0 | 0,8–2,5 | 0,5–2,0 | – | 1,5–2,5 | – | – | 0,15 | 0,25 | 0,15 | 0,015 | 0,05 | 0,10 |
| VT22 | Same | 4,4–5,7 | 4,0–5,5 | 4,0–5,5 | – | 0,30 | – | 0,5–1,5 | 0,15 | 0,5–1,5 | 0,18 | 0,015 | 0,05 | 0,10 |
| PT-7M | Same | 1,8–2,5 | – | – | – | 2,0–3,0 | – | – | 0,12 | 0,25 | 0,15 | 0,006 | 0,04 | 0,10 |
| PT-3V | Same | 3,5–5,0 | 1,2–2,5 | – | – | 0,30 | – | – | 0,12 | 0,25 | 0,15 | 0,006 | 0,04 | 0,10 |
| AT3 | Same | 2,0–3,5 | – | – | – | – | – | 0,2–0,5 | 0,20–0,40 | 0,2–0,5 | 0,15 | 0,008 | 0,05 | 0,10 |
Note. The sum of other impurities in all alloys is 0.30%, in the VT1-00 alloy - 0.10%.
Determination by spark
This is one of the most error-free methods for determining titanium. For it you will need a grinder or a sharpening machine, and if you don’t have one, any abrasive surface, for example, a fine file or asphalt, will do. When aluminum comes into contact with a rotating grinding wheel, the material is ground down with virtually no sparking. The contact of the abrasive and steel is accompanied by a stream of sparks, which range in color from light yellow to dark red.
Tatan, when friction against an abrasive surface or when cutting with a cutting disk, forms a stream of long sparks of bright white color. The fact is that this metal has the property of pyrophoricity, as a result of which small particles of the material formed during grinding or sawing ignite and sparkle in the air. These sparks are much brighter and hotter than those generated when processing steel, so they are white in color and create an increased fire hazard. Titanium powder is even used in pyrotechnics to produce bright pyrotechnic fountains.
Characteristics of titanium-based alloys, metal properties and applications
Titanium was originally named "gregorite" by British chemist Reverend William Gregor, who discovered it in 1791. Titanium was then independently discovered by the German chemist M. H. Klaproth in 1793. He named it titan after the Titans of Greek mythology - "the embodiment of natural strength." It was not until 1797 that Klaproth discovered that his titanium was an element previously discovered by Gregor.
Titanium is a chemical element with the symbol Ti and atomic number 22. It is a shiny metal with a silvery color, low density and high strength. It is resistant to corrosion in seawater and chlorine.
The element is found in a number of mineral deposits, mainly rutile and ilmenite, which are widespread in the Earth's crust and lithosphere.
Titanium is used to produce strong light alloys. The metal's two most useful properties are corrosion resistance and its hardness-to-density ratio, the highest of any metallic element. In its unalloyed state, this metal is as strong as some steels, but less dense.
Physical properties of metal
It is a strong metal with low density, quite ductile (especially in an oxygen-free environment), shiny and metalloid white. Its relatively high melting point of over 1650 °C (or 3000 °F) makes it useful as a refractory metal. It is paramagnetic and has fairly low electrical and thermal conductivity.
On the Mohs scale, the hardness of titanium is 6. According to this indicator, it is slightly inferior to hardened steel and tungsten.
Commercially pure (99.2%) titanium has an ultimate tensile strength of about 434 MPa, which is similar to common low-grade steel alloys, but titanium is much lighter.
Chemical properties of titanium
Like aluminum and magnesium, titanium and its alloys immediately oxidize when exposed to air. It reacts slowly with water and air at ambient temperatures because it forms a passive oxide coating that protects the bulk metal from further oxidation.
Atmospheric passivation gives titanium excellent corrosion resistance almost equivalent to platinum. Titanium is able to resist attack from dilute sulfuric and hydrochloric acids, chloride solutions and most organic acids.
Titanium is one of the few elements that burns in pure nitrogen, reacting at 800°C (1470°F) to form titanium nitride. Due to their high reactivity with oxygen, nitrogen and some other gases, titanium filaments are used in titanium sublimation pumps as absorbers for these gases. These pumps are inexpensive and reliably produce extremely low pressures in ultra-high vacuum systems.
Common titanium-containing minerals are anatase, brookite, ilmenite, perovskite, rutile and titanite (sphene). Of these minerals, only rutile and ilmenite are of economic importance, but even these are difficult to find in high concentrations.
Titanium is found in meteorites and has been found in the Sun and M-type stars with surface temperatures of 3200°C (5790°F).
Currently known methods for extracting titanium from various ores are labor-intensive and expensive.
Production and manufacturing
Currently, about 50 grades of titanium and titanium alloys have been developed and used. Today, 31 classes of titanium metal and alloys are recognized, of which classes 1–4 are commercially pure (unalloyed). They differ in tensile strength depending on oxygen content, with class 1 being the most ductile (lowest tensile strength with 0.18% oxygen) and class 4 the least ductile (highest tensile strength with 0.40% oxygen). ).
The remaining classes are alloys, each of which has specific properties:
- plastic;
- strength;
- hardness;
- electrical resistance;
- specific corrosion resistance and their combinations.
In addition to these specifications, titanium alloys are also manufactured to meet aerospace and military specifications (SAE-AMS, MIL-T), ISO standards and country-specific specifications, as well as end-user requirements for aerospace, military, medical and industrial applications.
A commercially pure flat product (sheet, slab) can be easily formed, but processing must take into account the fact that the metal has a "memory" and a tendency to bounce back. This is especially true for some high-strength alloys.
Titanium is often used to make alloys:
- with aluminum;
- with vanadium;
- with copper (for hardening);
- with iron;
- with manganese;
- with molybdenum and other metals.
Areas of use
Titanium alloys in sheet, plate, rod, wire, and casting form find applications in industrial, aerospace, recreational, and emerging markets. Powdered titanium is used in pyrotechnics as a source of bright burning particles.
Because titanium alloys have a high tensile strength-to-density ratio, high corrosion resistance, fatigue resistance, high crack resistance, and the ability to withstand moderately high temperatures, they are used in aircraft, armor, naval vessels, spacecraft, and missiles.
For these applications, titanium is alloyed with aluminum, zirconium, nickel, vanadium and other elements to produce a variety of components, including critical structural members, firewalls, landing gear, exhaust pipes (helicopters) and hydraulic systems. In fact, about two-thirds of titanium metal produced is used in aircraft engines and frames.
Because titanium alloys are resistant to seawater corrosion, they are used for propeller shafts, heat exchanger rigging, etc. These alloys are used in housings and components of ocean surveillance and monitoring devices for science and the military.
Specific alloys are used in oil and gas wells and nickel hydrometallurgy for their high strength. The pulp and paper industry uses titanium in process equipment exposed to aggressive environments such as sodium hypochlorite or wet chlorine gas (in bleaching). Other applications include ultrasonic welding, wave soldering.
Additionally, these alloys are used in automotive applications, especially in automobile and motorcycle racing where low weight, high strength and stiffness are essential.
Titanium is used in many sporting goods: tennis rackets, golf clubs, lacrosse shafts; cricket, hockey, lacrosse and football helmets, as well as bicycle frames and components.
Determination by mass
The lightest of these three metals is aluminum, the heaviest is steel. For example, a titanium plate will be one and a half times heavier than an aluminum one and two times lighter than a steel one. If there is nothing to compare the sample with, then you will have to use a mathematical method. The density of the metals in question is known to us and is:
- for titanium – 4.5 g/cm³
- for aluminum – 2.7 g/cm³
- for stainless steel 7.8 g/cm³
This is the mass per unit volume. All that remains is to weigh the product on precise scales and determine its volume. If the product has a complex shape, then it is easier to find out the volume using the Archimedean method. Dip the sample into a container of water and determine the required value by the volume of water displaced. All that remains is to calculate the density by dividing the mass by the volume, and then check whether it corresponds to the density of titanium.
Identification by mark on glass
This method is the easiest and is based on the ability of titanium to stick to glass during friction, that is, you can draw with it. The same effect is observed when running a sharp edge over a smooth tile, which is due to the high coefficient of friction of pure titanium. If stainless steel scratches a glass surface, then, to your great surprise, this hard metal does not damage the glass, but leaves a characteristic metallic mark on it, which can only be washed off with very strong hydrofluoric acid. Aluminum cannot damage glass or paint on it. It should also be noted that some titanium alloys also do not leave a pattern.
History of discovery
The history of the discovery of metal is associated with the names of several scientists:
- At the end of the 18th century, the German Martin Klaproth and the Englishman William Gregor simultaneously discovered the substance dioxide.
- Ten years later, their company was joined by the Frenchman Louis-Nicolas Vauquelin.
- By the mid-19th century, Jens Berzelius obtained the metal titanium.
- Another hundred years later, the Dutch isolated material of increased purity.
A rod consisting of titanium crystals of high purity
. The name of the new substance was proposed by Klaproth: according to the tradition established by him, the chemist named the discovered element after a character from Greek mythology.
The Titans are the children of the main gods of the Greek pantheon, Zeus and Gaia. That is, the second generation of gods.
Anodizing test
It is known that as a result of natural oxidation in air, a layer of TiO2 oxide is formed on the surface of titanium. By electrochemical method it is possible to obtain an oxide layer with a coloring effect. To carry out the experiment, you need a 12-volt car battery or several Krona batteries. The titanium sample is connected with a wire to the “+” electrode. Any iron rod wrapped in a piece of cloth soaked in saline solution or any other conductive liquid (vinegar, cola) is connected to the “-” electrode. If you run this cloth over a titanium surface, it will instantly become colored, which will never happen with stainless and aluminum products.
There are a number of other testing methods that allow us to distinguish real titanium from other metals that surround us in everyday life. For example, titanium objects seem warmer upon tactile contact. If you heat one part of the part, the other will remain cold, which indicates its low thermal conductivity. Cutting titanium is much more difficult than other metals. Material particles stick to the blade, making cutting unstable and unsafe. To accurately determine titanium, it is recommended to carry out not one, but several methods.
Let's talk about titanium or anything else you wanted to ask.
Titanium is a shiny, silver-colored metal that can be easily processed through various types of processing—drilling, turning, milling, and grinding. When sawing, drilling and milling titanium, it is necessary to constantly use a cooling lubricant, and do not put too much pressure on the tool; Titanium cannot be soldered, but is forged well (both hot and cold); before drawing titanium wire, it must be annealed. It has high strength, low density, and is quite lightweight.
In terms of corrosion resistance, titanium is comparable to precious metals.
Recently, in foreign countries, a wide range of different types of jewelry has been made from titanium. Titanium has become attractive for jewelry making due to the interesting color effects that form on its surface when heated.
This phenomenon is explained by the fact that when heated, an oxide layer is formed on the surface of titanium, which absorbs a certain amount of light, and only the remaining part of it is reflected in the form of a spectral color that we perceive.
With increasing annealing temperature, the oxide layer increases proportionally. As the thickness of the oxide film increases, more light is absorbed and a clearly demarcated range of tarnish colors is formed, ranging from light yellow (little light is absorbed in a thin layer) to greenish, violet and blue, down to dark blue (a thick layer reflects only a small part of the light) .
When making, for example, a bracelet, one end of the strip is heated with a narrow hot flame: the yellow tone that forms first slowly, which allows it to be observed, passes along the entire length of the strip, followed by greenish, violet and blue tones.
It is noteworthy that at high annealing temperatures, titanium once again turns yellow. If a strip painted in this way is bent into a ring, then both ends of the yellow color will differ in intensity. The same method can be used to make plates for brooches and pendants.
The color effect on a titanium plate can be enhanced by subsequent etching, for which a protective varnish is applied in the usual way and the pattern is scraped out, and then etched in a cold hydrofluoric acid solution. After etching, the gray color of the metal appears between the tarnish colors, successfully complementing and emphasizing the multicolored nature of the entire surface.
Thermal oxidation can be carried out using a muffle furnace or a conventional torch.
First, titanium acquires its first color – golden. As the temperature rises, various shades appear: from light yellow to greenish, purple and blue, all the way to dark blue. To obtain special effects on the surface, you can use various tonic additives, giving the products a very beautiful charcoal gray color.
Flame painting is done using a gas torch, which in this case becomes the artist's brush. Since precise color control is not possible, you must rely on your own artistic taste and approach. Any burner is suitable for use, since high temperatures are not required; A large, soft flame can produce patches of even color, while a small, hot flame can produce a rainbow of colors. Flame painting can also be carried out in a standard muffle furnace. By placing your jewelry in the oven for just a few minutes, you can achieve gold, purple, and blue colors. The heating temperature and residence time of the products in the oven in each specific case depend on the size and thickness of the decoration. This method can also be used to produce single-color paints.
Titanium can be painted more accurately using the electrolytic oxidation method. Depending on the voltage used, it is possible to obtain layers of different thicknesses and, therefore, different shades: yellow, dark blue, cyan, violet, blue-green. If it is necessary to obtain several color shades on one product, then the plate is processed first at the lowest voltage, and then the area on which this shade remains is closed, and the processing of the rest of the surface continues in the same way, but at a higher voltage.
Treatment can also be done in a different sequence: the highest voltage is applied first, the treated area is closed, and everything else is sandblasted. Colored layers obtained by electrolytic methods can be made shiny and also white by covering the corresponding areas and sandblasting others, or by applying a protective varnish and etching with hydrofluoric acid.
Sawing, drilling, drawing and soldering of titanium.
Titanium in some cases behaves differently than metals commonly used in jewelry.
When sawing titanium with a hacksaw, a light cut is first made, and only after the hacksaw blade has grasped the metal can the pressing force be increased.
Titanium can be processed with ordinary files without applying too much pressure, otherwise the file cut will become clogged and it will become “greasy”, which is why it needs to be cleaned from time to time.
When drilling, you should use lubricant and remember that the drill quickly becomes dull, and therefore requires new sharpening. When milling, the tool is subjected to heavy loads, so it must be cooled with oil. Turning, so that the cutter does not become dull quickly, should be performed at a low speed of the part; Processing with diamond and ceramic grinding wheels is recommended.
Titanium can be worked by pressure, but in this case intermediate annealing must be carried out frequently because it hardens quickly. When rolling, a lot of force is required.
When drawing wire, it is advisable to first anneal it - in this case, the lubricant (oil or soap) adheres better to the oxide film; Annealing should also be carried out after “passing” every third die. At a temperature of 650-950°C, titanium can be hot forged; it can also be processed in a cold state - in this case, it is more susceptible to stretching than compression.
Titanium cannot be soldered with either soft or hard solder, and welding is carried out only in a shielding gas environment. A jeweler can connect titanium parts only mechanically, for example, by riveting. Like all other metals, titanium can be bonded if the surfaces to be joined are large enough.
Surface treatment of titanium is carried out first with sandpaper of various grain sizes, and then polishing; A shiny surface is best achieved with nickel oxide paste or precious metal abrasives.
To prepare the surface of a titanium product for painting, it is recommended to lightly etch it: the product is momentarily dipped in a 2% hydrofluoric acid solution, then washed, and then treated with a regular sulfuric acid etching solution.
Materials used: ART MATERIALS SCIENCE. JEWELERY ALLOYS: TUTORIAL. Author/creator: Mutylina I.N.