29Jan
- By: Semantics
- Uncategorized
- Comments: 0
When manufacturing metal, the enterprise uses a classification of workpieces according to structural features.
Metallurgists usually observe changes in structure during metalworking, including after heat treatment. And one of these states is austenite, and after quenching followed by cooling, pearlite, martensite and other changes can be obtained. In this article we will talk about which steels belong to the austenitic class and what properties these materials have. This formation can be obtained in a steel billet, that is, in a solution of iron with the addition of carbon. The peculiarity of this state lies in the arrangement of the atoms of these substances. They sequentially form a pattern in one of two options:
- BCC A-Fe. This is a body-centered structure, according to which the atoms are arranged like this: they are at each vertex of the cube (there are 8 in total), and there is also one in the very center). This option does not happen often, on average in 10% of cases.
- FCC U-Fe. The volume of the structure is preserved, but the same number is added to the previous vertex points - they are placed in the center of each face. But there is no atom in the core. Thus, there are 16 of them in total. This is the most frequently appearing structure - face-centered. It is very strong in relation to low and high temperatures, as well as loads.
If we say what “austenitic steel” means simply, then it is a special structure of the metal that determines the technical characteristics of the alloy. When its state changes (heating, cooling, etc.), its properties also change. It is thanks to passing through austenite with subsequent cooling that such popular heat treatment as hardening is possible (heating above the critical point - until the crystal lattice changes). This procedure is popular because it is an excellent, inexpensive and fairly technologically simple way to increase the strength of metal.
This modification of the metal is characterized by a high degree of alloying (the most common alloying additive is chromium). Its peculiarity is the presence of a face-centered lattice, and the fact that it is preserved even in extreme cold. The main characteristics of austenites are strength and resistance to deformation even when heated. All this allows the use of products made from the material in the most dangerous, aggressive environments; they are very actively used in mechanical engineering, as well as in the chemical and oil industries.
Mechanical properties of austenitic steels
At the moment of crystallization, the metal goes through 1 phase, and after that the crystal lattice remains unchanged even when exposed to ultra-low temperatures, for example, -200 degrees. The alloy is based on iron and must be alloyed. The most commonly used alloying additives are nickel and chromium; other impurities are added in lower concentrations. Depending on how large the proportions of chemical metallic and non-metallic substances are, the characteristics change - chemical, physical, technological, and special properties appear.
Additives used in the alloying process are:
- Ferritizers. They stabilize the austenite structure and also increase the proportion of ferrite after cooling. They also predetermine the formation of a bcc lattice. These include the following elements: vanadium, tungsten, titanium, silicon, niobium, molybdenum.
- Austenizers. They expand the austenite region. Interestingly, there is even the term austenitization - this is special heating, as during hardening, followed by short-term holding and cooling.
Not all grades of the austenitic steel class have the same properties. Indeed, in addition to the heat treatment method, the composition is also important. Therefore, as in all other cases, when considering the structural varieties of alloys, the incoming components and proportions should be taken into account. We will note what properties are characteristic of some of the austenites:
- Stainless, corrosion resistant. The production of these popular steels is regulated by the regulatory document GOST 5632-2014. According to him, such compositions contain 18% chromium, 30% nickel and 0.25% carbon. There may also be various impurities (both beneficial and harmful), for example, silicon, manganese and molybdenum. Corrosion immunity is so valued that it is used everywhere - from the manufacture of household products to complex components in mechanical engineering. The substances react with oxygen and form an oxide film on the surface. It is this that is protective and is not disturbed even by strong temperature changes. Insusceptibility to heat is explained by its fairly low carbon content.
- Austenitic heat-resistant steels. They have a very high maximum heating point, so they can be used in complex moving units, as well as in direct contact with steam, fire and other hot objects. Temperatures up to 1100 degrees are absolutely not scary for them; it will not make significant changes in the deep structure of the material. This is explained by the fact that the alloy has an fcc lattice and additives such as boron, niobium, molybdenum, vanadium and tungsten. The listed impurities increase resistance to heat. Let's give an example of use - airplane turbines, all elements of a car's internal combustion engine, etc.
- Cold resistant. To achieve this effect, high-alloy steel should be made with a high concentration of nickel (25%) and chromium (19%). An interesting feature of these products is that high strength and ductility are maintained only in the cold, while at room temperature the characteristics can change in a negative direction.
Note that the composition of austenitic steel is expensive, since a large number of alloying components are added to it. Therefore, not all production areas can boast of the presence of austenite parts. The main impurities are chromium and nickel, and they are expensive.
This class of alloys is characterized by various controlled structural transformations, so you can get:
- Ferrite, if the composition is heated to ultra-high temperatures.
- Intercrystalline corrosion. They try to prevent this, since this process leads to internal destruction of the structure, deep layers and surface. The fact is that when iron heats up to more than 900 degrees, excess carbide phases appear, which, in turn, already affect corrosion transformations.
- Perlite. This is a commonly used metal structure that comes in the form of small grains and plates. Its formation is inevitable during slow, gradual cooling of the workpiece directly with the furnace to a temperature of 730 degrees. It is at this point that changes occur in the crystal lattice due to eutectoid decomposition. It is also called pearlite transformation. During this process, ferrite and cementite, which have a lamellar shape, simultaneously grow.
- Martensite. This is another type of structure, represented by plates in the form of needles or thin slats. It is formed when the temperature of the product is sharply reduced, for example, directly from the oven and into cold water or oil.
Thus, any transformations are planned in advance and controlled. Typically, the deciding factor in the procedure is the holding time and heating and cooling temperatures. This is determined by the content of carbon and other alloying additives. Those alloys that have the least amount of impurities crystallize faster.
Some elements of the iron-carbon diagram
Let's highlight several boundaries on the iron-carbon diagram:
- ACD line. Liquidus line . When the alloys below it are cooled, their crystallization begins;
- AECF line. Solidus
line . When cooling alloys below it, the entire alloy turns into a solid state; - ECF line. Sometimes called ledeburite transformation line. When alloys with a carbon content above 2.14% are cooled, the liquid phase below it turns into ledeburite;
- PSK line. Pearlite transformation line
. When alloys below it are cooled, austenite transforms into pearlite.
Let's note a few important points on the diagram:
- point E. The point of maximum saturation of austenite with carbon is 2.14%, at a temperature of 1147°C;
- point P. The point of maximum saturation of ferrite with carbon is 0.025%, at a temperature of 727 ° C;
- point S. Point “0.8% С-727°С” of transformation of austenite with a carbon concentration of 0.8% into pearlite (eutectoid) of the same average concentration;
- point C. Point “2.14% С-1147°С” for the transformation of a liquid with a carbon concentration of 2.14% into ledeburite (eutectic) of the same average concentration.
Often the temperature values at which structural changes occur in a particular alloy are denoted by the letters A:
- A1 – PSK line;
- A2 – line MO – Curie point, at which a change in the magnetic properties of alloys occurs;
- A3 – temperatures corresponding to the GS line;
- Acm – temperatures corresponding to the SE line.
Since the temperatures of phase transitions during heating and cooling are slightly different, additional letter designations are often introduced:
- с – for temperatures of phase transitions during heating;
- r – during cooling,
for example Ac1 or Ar1.
Methods for producing austenitic carbon steels
The entire initial process can be described as follows: in order to obtain austenite, it is necessary that grains begin to appear and grow in the initial structure of the alloys. First, the grain size changes at the surface during the phases of the appearance of carbides; over time, the entire thickness of the workpiece changes its structure.
The second method of producing austenite is heating the pearlite modification of iron to 900 degrees (after eutectoid decomposition). This alloy consists partly of cementite and the other part of ferrite. For such a transformation to occur, a minimum carbon content of the steel is required - no less than 0.66% substance content. After the temperature increases by more than 900 degrees, the ferrite structure transforms into austenite, and the cementite structure completely dissolves. It turns out to be excellent quality stainless steel.
There is another option - with a titanium mixture. In such cases, a metal blank is taken and placed in an induction furnace, in which a vacuum is maintained. In it, a high heat is first achieved, and then it is maintained for a long period. During this time, diazotization occurs, that is, the removal of nitrogen atoms from the steel melt. The time period is determined individually depending on the mass of the workpiece. Titanium and other metallic and non-metallic impurities are then gradually added to form nitrides in reaction with iron.
But the main method for producing austenitic steel is based on the creation of a high-alloy chromium-nickel alloy. The product can be alloyed by adding chromium and nickel. After the substances are added to a tight solution, you need to maintain a high temperature for a long time, this gives:
- corrosion resistance;
- strength;
- heat resistance;
- increased release of carbides.
And if you add molybdenum and phosphorus, you can achieve increased viscosity and fatigue strength.
Chemical elements and their effect on austenite
Like any alloy steel, this one can be based on a number of alloying additives. Let's see how their content in the melt affects the basic qualities of the metal:
- Chromium. Its high concentration, exceeding 13% (but not more than 19%), contributes to the creation of an oxide film. It is known to prevent corrosion. It is interesting that this effect of chromium is only relevant at low carbon content. Because otherwise, these two elements begin to react, forming carbide, which, on the contrary, accelerates the rusting process.
- Nickel. Another constantly used material. There can be a lot of it, even more than 50%. But in order to obtain austenite from iron, only 9-12 percent is enough. The chemical has a very positive effect on plasticity - it becomes higher. In addition, the grain size becomes smaller, which has a good effect on strength.
- Carbon. Hundreds and tenths are usually added. This is enough to increase strength. This is due to the fact that the substance leads to the formation of carbides.
- Nitrogen. It replaces carbon if it cannot be added to the alloy for some reason, for example, if the product must be resistant to electrical and chemical influences.
- Bor. It increases plasticity very well, even if the substance is in very small quantities, and the grain becomes smaller.
- Silicon and manganese. Added to stabilize austenite and also to increase strength.
- Titanium and niobium. Used in the manufacture of cold-resistant alloys.
Reading an Iron-Carbon Diagram
We can see the composition of an alloy with a given initial carbon content at a given temperature by moving along a vertical line corresponding to the carbon content in the alloy.
Consider, for example, the AEC region. It is adjacent to areas of austenite AESG and the liquid phase. The alloys in it consist of a liquid phase and the resulting solid austenite. How to determine the carbon concentration in different phases for a given alloy? Let us consider, as an example, an alloy with an initial carbon concentration of 2.5% at a temperature of 1250°C.
Let’s draw a horizontal straight line from this point on the graph “2.5% C – 1250°C”. The intersection of this straight line with the AE line bordering the austenite region will show the carbon concentration in austenite at a given temperature (~1.5%).
The intersection of the same horizontal line with line AC, bordering the liquid phase region, will show the concentration of carbon in the liquid phase at a given temperature (~3.5%).
This is how we can determine the carbon concentration in the phases of any alloy at a given temperature:
- in the liquid phase and austenite in the AEC region;
- in the liquid phase in the CDF region (carbon concentration in cementite, of course, is constant - 6.67%);
- in austenite in the SEFK region;
- in ferrite in the QPKL region;
- in ferrite and austenite in the GPS region.
As we can see, at a carbon concentration above 2.14%, the saturation of the cooled melt with carbon always tends to 4.3% (along the AC and DC lines) as it approaches a temperature of 1147°C (ECF level). Next, the liquid transforms into ledeburite (eutectic). Naturally, with the same average carbon content.
As the temperature approaches 727°C (PSK level), the carbon concentration in austenite (“free” and/or included in ledeburite) tends to 0.8% (according to the GS and ES lines). Next, the transformation of austenite into pearlite (eutectoid) occurs. Perlite, of course, has an average carbon content of 0.8%.
Application of austenitic steels
Most common use:
- Any elements that are used at high temperatures - more than 200 degrees (up to 1100). These could be aircraft turbines or various parts in an engine. However, you should carefully monitor what chemical reactions will occur upon contact with fuel, steam and other aggressive media. Sometimes cracks appear. To prevent this possibility, impurities such as vanadium and niobium should be added. A carbide phase will be formed with them, due to which the surface is hardened.
- Various mechanisms that are subject to rapid temperature changes. For example, when welding certain materials.
- Electrical equipment, contacts. They can be made due to the fact that austenite is resistant to electromagnetic waves.
- Parts for devices operating in aquatic environments or in conditions of high humidity. This is possible due to its corrosion resistance. Nickel and chromium, which contribute to this characteristic, also prolong the wear of the element.
Austenitic steel grades
All classes can be divided into three categories:
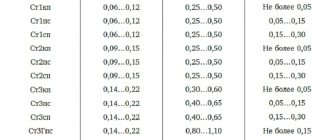
- Corrosion-resistant: 08Х18Н10, 12Х18Н10Т, 06Х18Н11 (they contain chromium and nickel), 10Х14Г14Н4Т, 07Х21Г7АН5 (with the addition of manganese), 08Х17Н13М2Т, 03Х16Н16ьЗ (a special feature is the presence of molybdenum), 02Х8Н22С6, 15Х18Н12С4Т10 (they contain a lot of silicon).
- Heat-resistant, for example, 08Х16Н9М2, 10Х14Н16Б, 10Х18Н12Т, 10Х14Н14В2БР. A special feature is the presence of boron, tungsten, niobium, vanadium or molybdenum in them.
- Cold-resistant: 03X20N16AG6 and 07X13N4AG20, they contain a lot of chromium and nickel.
Pay attention to the marking, it is determined by the regulatory document, more about it below.
GOST 5632-2014
This document dictates the requirements for each specific brand. The tables presented there list the qualities and indicators that are responsible for the final result - strength, wear resistance, etc. Let's look at the marking and note that it combines numbers and letters. The letters indicate the alloying additive that is present in the greatest quantity (the smallest impurities may not be displayed in the name, but will be listed in the technical data sheet of the alloy). At the very beginning there is only a number - these are hundredths of carbon. Then the letter of the additive followed by clarification - how many percent. Let's look at a simple example. 06Х18Н11, in this brand:
- 0.06% carbon;
- 18% chlorine;
- 11% nickel.
Let's present a table of elements that are contained in the most common brands:
Heat treatment features
Despite the fact that this material has increased strength characteristics, it is very difficult to process metal. Typically, to improve the quality of the workpiece, one of the following methods is used:
- Annealing. This process involves heating to high temperatures (changing the crystal lattice) followed by holding for several hours. After this, cooling occurs in one of the following ways: in oil, water, or in air at room conditions. This helps to reduce the hardness of austenitic steels.
- Double hardening. Repeated heating procedure increases the heat resistance of the material. Additionally, aging is often used.
Austenite is a very commonly used alloy. To understand the topic in more detail, watch the video:
Heat treatment of steels at KVADRO LLC
Our company has been producing custom heat treatment of metals in St. Petersburg for almost a quarter of a century.
We carry out heat treatment of steels (including stainless steel, tool steel, etc.) according to the Customer’s drawings or specified conditions, as well as other metals and alloys (aluminum and titanium, brass and bronze, etc.).
The main types of heat treatment of metals carried out at our enterprise to order:
- hardening (including in salt baths, for example, for hardening high-speed cutters);
- vacation;
- annealing;
- normalization;
- improvement;
- cementation.
We also remind you that with us you can use a wide range of metalworking methods, including milling and turning.