General information about martensite
The structure based on a supersaturated solid solution of carbon in iron is called martensite.
It is obtained by supercooling the austenite phase. In other words, martensite is the result of hardening steels with a carbon content above 0.3%. Martensite crystals have a tetragonal structure, where iron atoms occupy space at lattice sites. In appearance, martensite consists of multiple dark iron needles on a light background. The angle of inclination of these needles is on average 60 degrees relative to each other. It is impossible to detect traces of carbon on the surface of martensite, since it is completely in a dissolved state.
Martensite is distinguished by its strength compared to other phases. Mechanical properties up to a certain point are directly dependent on the amount of carbon in the steel. But it is worth noting that after passing a certain point, the strength drops and fragility begins to increase.
According to research conducted in the 30s of the last century by Soviet scientists, the reasons for the high mechanical characteristics of martensite lie in the following:
- The structure of martensite is blocky in nature, despite the fact that the blocks themselves are quite small in size.
- Resistance to static distortion, which means the stability of the position of atoms when they are displaced from the ideal arrangement of atoms in the crystal lattice.
- When exposed to mechanical loads, and as a result of plastic deformation, tiny solid particles are released, blocking the sliding of the layers relative to each other and increasing the hardness of the alloy.
The hardness of martensite has a volatile character and depends on the heating, cooling and holding time of the steel. On average, its value ranges from 35 to 70 units on the Rockwell scale. Martensite also has a large specific volume. Its value is higher compared to other phase structures such as austenite, pearlite, etc.
As a consequence of all of the above, the formation of martensite is accompanied by significant changes in steel volume. This, in turn, leads to an undesirable increase in internal stress in the structure, which can cause cracks in the future.
Martensite: how and why
The most remarkable property of steel is its ability to be hardened to high levels of strength by simple hardening. Tempering steel usually occurs by immersing the heated metal in a coolant such as water, oil, or liquid salt. To increase strength, it is necessary that this heated steel contain austenite, or better yet, be completely austenitic. Then very rapid cooling will not give austenite the opportunity to transform into the thermodynamically “favorable” ferrite + cementite structure. Instead, a new structure is formed, called martensite. This martensitic phase gives the steel a very high level of strength.
Carbon: a lot in austenite - little in ferrite
As is known, austenite has a face-centered cubic crystalline (fcc) structure, ferrite has a body-centered crystalline (bcc) structure. The phase diagram of steel shows that the fcc structure - austenite - will dissolve much more carbon than the bcc structure - ferrite. At temperature A1, the amount of carbon that can dissolve in austenite is 38.5 times (0.77/0.02 = 38.5) greater than in ferrite.
The fact is that carbon atoms are much smaller than iron atoms. Dissolved carbon atoms are located in spaces between relatively large iron atoms. The bcc structure is able to "absorb" more carbon atoms because some of the spaces between the atoms in this structure are significantly larger than any gaps in the fcc structure.
Slow cooling of austenite – ferrite plus cementite
Figure 1 shows a diagram of the transformation of austenite in steel with a carbon content of 0.60% to ferrite. The vertical line represents the transformation front, which moves from left to right. After this front has advanced, for example, 25 mm, in this 25 mm long region the carbon content should drop from 0.6% to 0.02%. With slow cooling, carbon can have time to move ahead of the transformation front in austenite along the direction shown by the dashed arrow due to the diffusion mechanism.
Figure 1 – Scheme of the advance of the front of transformation of austenite into ferrite
Rapid cooling of austenite – martensite
However, if this transformation is forced to occur very quickly by quenching, there will no longer be time for the diffusive movement of carbon atoms. Therefore, some of them - or all of them - will remain in ferrite. This excessive carbon content in ferrite leads to a sharp distortion of its bcc structure - as a result, a martensitic structure appears.
Atomic lattice: from ferrite to martensite
Figure 2 shows side by side the atomic cell of bcc ferrite and the distorted atomic cell of martensite. The atomic cell of martensite is similar to the bcc cell of ferrite in that it also has an atom at the center and an atom at each of the eight corners. However, this atomic cell is no longer a cube. One of its sides, which is called the lattice period c or face c (see Figure 2), is longer than the other two, which are called periods a or faces a. This crystal structure is called body-centered tetragonal (BCT).
Figure 2 – Comparison of the crystal structures of ferrite and martensite
More carbon - higher hardness
Figure 3 shows how, as the amount of dissolved carbon in martensite increases, its c face becomes larger and larger compared to its a face. Increased carbon content in martensite is achieved by quenching austenite with a higher carbon content. The graph in Figure 3 shows that with increasing carbon content, the distortion of the atomic lattice from cubic - face c - becomes increasingly greater compared to face a. This occurs due to carbon atoms embedded in the bct martensite lattice.
Figure 3 – Dimensions of faces a and c of a body-centered martensite cell (1 nm = 1000 µm)
The strength and hardness of martensite increases very strongly with increasing carbon content, as can be seen from Figure 4.
Figure 4 – Rockwell hardness of freshly quenched martensite depending on carbon content
The following interpretation helps to understand why the strength of martensite increases with increasing carbon content. It's convenient to think of the chemical bonds that hold the iron atoms together as springs. As the carbon content increases, these springs stretch to accommodate additional carbon atoms in the lattice. And in order to stretch these stretched springs further - to deform the martensite - more and more effort is required.
Structure and properties
The crystal structure of martensite is tetragonal, the unit cell has the shape of a rectangular parallelepiped, iron atoms are located at the vertices and center of the cell, carbon atoms are located in the volume of the cells. The structure is nonequilibrium, and there are large internal stresses, which largely determines the high hardness and strength of steels with a martensitic structure.
When heating steels with a martensitic structure, a diffusion redistribution of carbon atoms occurs. Two phases appear in steel: ferrite, which contains very little carbon (up to 0.02%) and cementite (6.67% carbon). The unit cell of ferrite has the shape of a cube, the iron atoms are located at the vertices and in the center of the cube (body-centered structure), cementite has a rhombic structure. The unit cell of cementite has the shape of a rectangular parallelepiped.
The crystal lattice of martensite is connected by constant crystallographic relationships with the lattice of the initial structure of austenite, that is, planes with certain crystallographic indices in the martensite structure are parallel to planes with certain indices in the austenite structure. The relationship between the crystallographic directions in the lattices of martensite and austenite is similar.
Properties of martensite
A characteristic feature of martensite is its high hardness and strength. The more carbon there is in steel and, naturally, in martensite, the greater the degree of tetragonality (distortion) of its crystal lattice, the greater the resistance to plastic deformation, and therefore the higher the hardness (Fig. 5.14) and strength.
When the carbon concentration in steel increases to 0.6...0.7% or more, the hardness increases to 65...66 HRC. The tensile strength (tensile strength σв) of martensite at the same carbon concentration reaches 2400...2600 MPa. This is 2.5 times higher than the strength of low-carbon martensite containing 0.015% carbon (up to 1000 MPa). At the same time, martensite has low plasticity. As the carbon content increases, its tendency to brittle fracture increases; in tensile tests, steels fail brittlely even with a carbon content of more than 0.35%.
Martensite has the highest specific volume compared to austenite and other steel phases. Therefore, the martensitic transformation occurs with an increase in volume, which is one of the main reasons for the occurrence of significant stresses during hardening of steels and, as a consequence, deformation of steel products or even the formation of cracks.
Properties and structure of martensite
Martensite is a needle-shaped grain in the microstructure of a metal, representing a supersaturated solid solution of carbon in alpha iron. This structure is typical for steels that have undergone a hardening procedure, as well as for some pure metals that have polymorphism. Martensite owes its name to Adolf Martens, a German scientist who devoted most of his life to the study of metals and their properties. It should be noted that martensitic steels, due to the characteristics of their structure, are distinguished by the highest hardness among similar materials.
Microstructure of martensite
The phenomenon of martensitic transformation, which occurs during heating and cooling of steel, is associated with the unique effect of “metal memory”, discovered and described by scientists G.V. Kurdyumov and L.G. Khandros in 1949. The essence of this effect is that the deformation of the metal created in it at the moment when the direct martensitic transformation occurs completely disappears during the reverse transformation. Thanks to this effect, scientists were able to create alloys that have memory of their shape. Products made from such alloys, which have been subjected to deformation in the martensitic state, return to their original shape if they are heated to a temperature that causes the martensitic transformation in steel.
The crystal lattice of martensite formed in the structure of a hardened metal is not cubic, but tetragonal. Each element has the shape of a rectangular parallelepiped. The central part of such a cell (as well as its vertices) is occupied by iron atoms, with carbon atoms in the internal space between them.
Martensitic steels, as mentioned above, are distinguished by high hardness and strength, and this is explained by the fact that the structure of martensite, being nonequilibrium, is characterized by the presence of strong internal stresses. In martensitic steels, when heated, carbon atoms are redistributed. This phenomenon is of a diffusion nature. As a result of this distribution, two phases are formed in the steel structure, each of which differs in carbon content and the shape of its crystal lattice.
Crystal lattice of martensite
These phases that characterize all martensitic steels when heated are:
- ferrite, which contains a very small amount of carbon - up to 0.02% (the elementary cells of the ferrite crystal lattice have the shape of a cube, the tops and center of which are formed by iron atoms; the rest of the space in such cells is occupied by carbon);
- cementite, in which the carbon content is much higher - up to 6.67% (the rhombic crystal lattice of cementite is formed by elementary cells shaped like a rectangular parallelepiped).
The initial structure for the formation of martensite is austenite. The crystal lattices of these formations, simultaneously present in the microstructure of steel, are interconnected by orientation relationships. This connection lies in the fact that the lattice planes of austenite and martensite, which have certain crystallographic indices, are parallel to each other.
Martensite, which forms the microstructure of steels, can be present in two forms.
Various types of martensite formed when austenite is quenched
Lamellar (twinned) martensite
This structure is formed at temperatures below 2000. It is characteristic of carbon and alloy steels. The properties of martensite of this type, present in the structure of the metal in the form of plates, are determined by the presence on such plates of the so-called midrib - a middle line characterized by increased etchability. This martensite is called twin martensite because the midrib of each of its plates is formed by many twins. Such twins, located along the planes of martensite plates, have a thickness of 5–30 nm.
Optical micrograph of martensite of a lamellar structure
Lath (dislocation) martensite
This formation is characteristic of the structure of high-alloy, low- and medium-carbon steels. The temperature threshold at which the formation of a martensitic structure occurs in such steels is above 3000. Martensite of this type, in full accordance with its name, has the shape of laths elongated in one direction, the thickness of each of which is in the range of 0.2–2 μm (at this means their length is approximately 5 times greater than their width). The structure of the metal formed from martensite of this type is presented in the form of a combination of groups (packets) of such lath crystals parallel to each other. In this structure, one can also see layers between the martensite laths, consisting of retained austenite. The thickness of such layers in various types of alloys can range from 10 to 20 nm.
Optical micrograph of lath structure martensite
Under certain conditions (in particular, when the temperature range between the beginning and end of the martensitic transformation is too large), martensite of both types can form in steels. High temperature leads to a decrease in the strength of austenite, therefore the structure of martensite, which is formed in the alloy, has a lath shape. With decreasing temperature, when the strength of austenite increases, lamellar-type martensite is formed in the steel.
There is a certain category of low-carbon steels in which there is practically no retained austenite, and the formed martensite has only a lath shape. The temperature at which martensitic transformations are observed in such steels is about 4000 C.
Martensitic steels
Martensitic steels include high-alloy steels, the structure of which, after heat treatment, is martensite.
The martensitic alloy itself is difficult to cut. Its workability is increased by pre-annealing at a temperature of 800-900 ºC.
As a rule, martensitic steels are alloyed with metals such as tungsten, nickel and molybdenum to increase the heat resistance and corrosion resistance of the alloy to aggressive environmental influences.
Martensitic steel also has such a useful property as self-hardening, i.e. a spontaneous increase in hardness after heat treatment.
Martensitic steels belong to weldability group 3. Welding requires preheating to 200-300 ºC and subsequent annealing of the part. All this is necessary to reduce internal stress and reduce the likelihood of cracks forming on the surface of the weld. In practice, these steels are welded using argon arc and electroslag welding.
The mechanical properties of martensite-based steels are quite high. Thus, grade 15X5, used in the manufacture of high-pressure vessels, has a tensile strength of almost 400 MPa.
Additional alloying with tungsten and vanadium greatly increases the heat resistance of the alloy. The tensile strength of 10ХМФБ steel is already 600 MPa. Steel is used in the production of manifolds, pipelines and heating boilers.
An increase in the beryllium content in martensitic steels contributes to a further increase in their mechanical properties. The tensile strength of steel 12Х11В2МФ is approximately 850 MPa. Such grades are used in the production of parts experiencing increased thermal and mechanical loads. For example, in the casing and rotor of gas and steam turbines, and also as a material for blades of turboprop compressors.
Martensitic steels are quite elastic and resist shock loads well. Impact strength ranges from 80-150 Jcm2. Its value largely depends on the type of heat treatment and the content of certain elements. Its greatest value is obtained as a result of hardening followed by high tempering.
Martensitic steels do not have a high ductility value. The relative specific compression is 14-24%. This parameter depends to a large extent on the amount of carbon in the steel. Also, elements such as nickel and copper have a negative effect on the ductility of the alloy.
Theory of production of martensitic steels
During hardening of carbon steel containing more than 0.25 - 0.3% carbon, a sharp change in its properties is observed. The steel acquires a martensite structure. At a certain heating temperature and subsequent cooling, martensite crystals form from austenite grains.
This is interesting: Slings
The basis of the polymorphic martensitic transformation is the diffusion-free mechanism of transformation of gamma-iron austenite with a face-centered cubic crystal lattice (FCC) of austenite into alpha-iron with a body-centered lattice (BCC) of martensite.
The recrystallization process occurs at high, almost subsonic speeds, due to the coherent connection of growing martensite crystals with the original austenite crystals. The greater the structural and dimensional correspondence, the thinner the martensite needles.
The martensitic structure is a supersaturated solution of carbon in alpha iron; its crystals are prismatic in shape. The concentration of carbon and alloying elements determines the increase in the length of the prism and the decrease in its base, and, accordingly, the increase in the strength and hardness of martensitic steel. Due to the high elasticity and low mobility of atoms, the martensitic transformation occurs through cooperative coordinated displacement of atoms to distances less than interatomic ones. The newly formed martensite phase is a nonequilibrium system.
Due to the lamellar (needle-like) shape of the crystals and the plastic deformation (phase hardening) that occurs as the mismatch in the positions of the atoms increases and the coherence is broken, martensitic structures have higher strength, hardness and lower ductility compared to the original crystals of the austenitic structure. There are hypotheses about the wave nature of the process of plastic deformation.
Education
The physical mechanism of martensite formation is fundamentally different from the mechanism of other processes occurring in steel during heating and cooling. Other processes are diffusional, that is, atoms move at low speed, for example, with slow cooling of austenite, nuclei of ferrite and cementite crystals are created, additional atoms are attached to them as a result of diffusion, and, finally, the entire volume acquires a pearlite or ferrite-pearlite structure. The martensitic transformation is diffusion-free (shear transformation), atoms move at high speed along a shear mechanism, the propagation speed is about a thousand meters per second.
Features of martensitic transformation in steels
The condition for such a phenomenon as martensitic transformation is not a fixed temperature, but a certain temperature interval. The upper limit of this interval corresponds to a temperature that is several hundred degrees lower than the temperature at which austenitic decomposition begins. The end of this process occurs at a temperature that is significantly lower than room temperature. Such conditions for the formation of martensite are due to the fact that retained austenite is also present in the structure of the alloy.
The amount of martensite in the steel structure can be increased by subjecting the alloy to plastic deformation. This must be done at the temperature required for martensitic transformation. Austenite can transform into martensite even if the alloy is subjected to plastic deformation at room temperature.
Scheme of changes in martensite during heating
The formation in question in the steel structure can take a form called tempered martensite. The conditions for its formation are heating the alloy to a temperature that is lower than the temperature at which ferrite transforms into austenite. A characteristic feature of the process by which tempered martensite is formed is that the martensite, which has a needle or plate shape, is transformed into carbide inclusions of a spherical configuration.
The essence of transforming the initial structure of an alloy into a martensitic one is that the molecules in the crystals of such an alloy begin to move in an orderly manner, changing their location relative to each other and, accordingly, forming crystal lattices of a new configuration. Thus, there is no destruction, but only deformation of the crystal lattice cells, which leads to the formation of a new alloy structure.
Formation of martensite crystals in austenite grains
For the martensitic transformation of the structure of the alloy, during which there is not destruction, but a modification of the crystal lattices of the cells that form its structure, a very small amount of energy is required. This contributes to the fact that such changes occur at a high speed. The results of such transformations, as well as the conditions for their occurrence, make it possible to effectively change the characteristics of the alloys in which they occur, using thermal or mechanical methods.
Martensitic transformation
Martensite forms only in austenite environments. The reason why this transformation occurs is the presence of a large amount of free energy in austenite. The catalyst for the transformation process is temperature, which, depending on the chemical composition of the steel, should be at the level of 500-700 ºC.
It has also been proven that the martensitic transformation is closely related to crystallization centers that form with increasing temperature. They stimulate crystal growth, densifying the atoms and, accordingly, increasing the strength properties of steel. This process does not require a lot of energy and is activated at a fairly low temperature.
Crystal growth occurs as long as any of the atomic layers enters both the martensitic and austenitic crystal lattice. Moreover, there should be no dividing surface between these structures.
Otherwise, a shift of one phase relative to the other is formed, which causes the appearance of a significant amount of voltage at their boundary. Tension provokes the appearance of elastic deformations, as a result of which the crystals (needles) stop their growth.
During the transformation of austenite into martensite, no new chemical compounds are formed. This process is structural. Atoms change their location, which affects the type and size of the crystal lattice.
Martensitic transformation requires constant supercooling. It is also worth noting that the increase in the volume of the structure does not occur due to the growth of individual needles, but due to the formation of new, smaller in terms of crystal size, martensite.
Among the features of the martensitic transformation, it stands out that austenite cannot completely transform into martensite. There are exceptions - steels, the transition point of austenite to martensite lies below zero. But in most cases, there is always a certain volume of austenitic phases that have not undergone their structural changes. This is due to the physical properties of iron and carbon.
The transformation of austenite into martensite is one of the basic structural changes not only in steels, but also in alloys based on titanium and copper.
Types of martensite
Depending on the degree of heating and the temperature of the cooled medium, different types of martensite are obtained. There are the following main types:
- Lamellar martensite
- Lath martensite.
Each of them has its own characteristics and, accordingly, mechanical properties.
Lamellar martensite is observed mainly in high-carbon structural steels. It is formed as a result of hardening and is characterized by the presence of martensite in the form of plates. The tensile strength of such martensite can reach up to 900 MPa. Hardness up to 75 HRC.
Lath martensite is obtained as a result of tempering (hardening with high tempering) of alloy steels. The structure of this type has the shape of laths up to 2 microns in size. This type of martensite is characterized by greater wear resistance and better dynamic viscosity.
Subject to a certain temperature regime, the steel structure can contain martensite of both lath and plate type.
Application area
Having specific, and in some cases unique properties, martensitic steels are used for the manufacture of parts operating in difficult technical and chemical conditions. They are made from:
- elements of gas and steam turbines (in particular rotors, diaphragms, blades and casings);
- parts of welding machines;
- high pressure vessels, which must withstand 16 MPa;
- components for high pressure pumps;
- springs capable of withstanding heavy loads;
- individual parts of boilers, pipelines, manifolds through which high-temperature liquid or steam passes;
- instruments for various purposes (cutting, measuring, processing);
- medical instruments and individual parts of equipment.
The main disadvantages of such steels are: difficulties arising during machining and welding of individual parts. To solve the second problem, it is necessary to create special conditions for welding.
Magnetic properties of martensitic steel
The martensitic structure of the steel lattice has pronounced magnetic properties. Martensite is a ferromagnet in its pure form. However, maintaining an ideal chemical composition is difficult. Carbon martensitic steels alloyed with molybdenum, cobalt and chromium (EX9K15M2), cobalt and chromium (EX5K6), chromium (EX3) can be classified as hard magnetic materials.
Alloying with cobalt is most effective from the point of view of magnetism - cobalt atoms have a magnetic moment, thus, the residual induction of martensite increases. Low price and ease of mechanical and heat treatment makes it possible to use martensitic steels in magnetic systems as switches for changing direction when supplying control signals.
Weldability of martensitic steels
Welding technologies for martensitic alloys are complicated by the increased fragility of the metal after hardening. These types of steel are cooked after preheating from approximately 200 to 450 degrees, the ambient temperature should not be negative. Typically, parts made of martensitic steel are welded using manual arc welding methods using electrodes coated with special compounds. Sometimes other types of welding are used: argon arc, electroslag, submerged arc.
Application of martensitic steel grades
Alloying elements are added to martensitic steels to obtain the desired properties of the alloys: strength, wear resistance, cold-heat resistance, corrosion resistance. One grade of alloy steel can contain up to 7 alloying elements. Steels are alloyed with nickel, chromium, nitrogen, tungsten, beryllium, vanadium, silicon, molybdenum, copper, and boron.
Usually, the designation of steel encodes alloying additives and their quantity (38ХН3МФА), some experimental ones are encrypted with the letter E. In this case, the letter does not reflect the composition of the steel - EI, EP3. Sometimes steels intended for the manufacture of aircraft and automobile exhaust valves are abbreviated as silchrome.
Alloyed martensitic steels are able to withstand aggressive environments: acids, alkalis, salts, aggressive gases. According to their application, martensitic steels are classified as corrosion-resistant, heat-resistant, heat-resistant and special-purpose steels.
Corrosion-resistant steel grades (15Х28, 20Х13, 12Х18Н9) are used in pilot production and in the chemical industry.
Heat-resistant steel grades (KhN60Yu, 12Kh25N16G7AR, (15Kh6SYu)) are used for the manufacture of parts that operate under moderate loads at temperatures up to 1000 degrees.
Products made from heat-resistant steel grades (15Х6СУ, 08Х13, 14Х17Н2) can operate under load for a very long and long period at high temperatures.
Special steels include steels from which armored sandwiches are rolled. A special place is occupied by Hadfield steel (1.1% carbon, 13% magnesium). When working under high pressure conditions, spontaneous plastic deformation occurs and, accordingly, the degree of its strength increases. The unique mechanical properties have not yet been fully studied.
Magnetic properties of martensitic steel
The martensitic structure of the steel lattice has pronounced magnetic properties. Martensite is a ferromagnet in its pure form. However, maintaining an ideal chemical composition is difficult. Carbon martensitic steels alloyed with molybdenum, cobalt and chromium (EX9K15M2), cobalt and chromium (EX5K6), chromium (EX3) can be classified as hard magnetic materials.
Alloying with cobalt is most effective from the point of view of magnetism - cobalt atoms have a magnetic moment, thus, the residual induction of martensite increases. Low price and ease of mechanical and heat treatment makes it possible to use martensitic steels in magnetic systems as switches for changing direction when supplying control signals.
Weldability of martensitic steels
Welding technologies for martensitic alloys are complicated by the increased fragility of the metal after hardening. These types of steel are cooked after preheating from approximately 200 to 450 degrees, the ambient temperature should not be negative. Typically, parts made of martensitic steel are welded using manual arc welding methods using electrodes coated with special compounds. Sometimes other types of welding are used: argon arc, electroslag, submerged arc.
Martensitic transformations in polymorphic crystals
Similar martensitic transformations, when atoms do not change places, but only shift relative to each other at distances smaller than interatomic ones (shortening interatomic bonds and changing the angles between them), are observed not only in iron alloys, but also in other polymorphic crystals.
Such transformations, also called metamorphoses, take place in steels, pure metals: iron, cobalt, titanium, lithium, at least 35 metals, in solid solutions based on them, in semiconductors and polymers, in intermetallic compounds.
In contrast to normal equilibrium polymorphic transformations, martensitic transformations are diffuseless and metastable. These transformations are of a non-equilibrium nature. The physics of metals says: nonequilibrium states must be self-organized.
From the point of view of the second law of thermodynamics, martensitic transformations in substances occur with a decrease in entropy. This means that the crystal structures of such transformations are the result of self-organization, and their parameters approach supercritical ones.
The structure of the intermetallic nickel monoaluminide after martensitic transformation is capable of withstanding temperatures up to 1300 degrees under high loads, but due to increased fragility it is used only as a heat-resistant coating for gas turbine engines.
Some intermetallic compounds with martensitic structures containing platinum are used as catalysts in the production of nitrogen. In connection with the tightening of environmental standards for cars, developments are underway to burn combustion products using intermetallic compounds.
This is interesting: What is scraping? Features and where is it used?
On crystals of some semiconductors (silicon, germanium) one can observe direct or reverse diffuseless phase transitions of states. Experiments on heat treatment of silicon wafers were implemented in production with a 20% economic effect.
By studying the process of reversibility of martensitic transformations on recrystallization of the TiNi alloy (intermetallic compound), a change in the size of the samples was discovered.
Memory effect
Further experiments with various materials showed that many polymorphic crystals can exhibit properties such as shape memory effect, superelasticity and superplasticity.
Deformation and its reduction or even complete restoration of the original shapes during the reverse occurrence of martensitic transformations is called the shape memory effect. And all phenomena associated with martensitic transformations in substances are united under one name “unusual physical and mechanical properties.”
The shape memory effect is already used today in hydraulic couplings in shipbuilding and aviation, in damping devices, in thermal relays, in medicine for the treatment of scoliosis, joining broken bones, in heart surgery, and in dentistry.
Martensitic points
The main characteristic of alloys under a certain hardening regime is martensite points.
The temperature at which martensitic transformations begin is designated Mn. When the steel cooling temperature reaches Mn, an instantaneous avalanche-like process of steel recrystallization begins. Temperature Mn is determined for each steel grade experimentally at metallurgical enterprises. The Mn value decreases with an increase in the amount of carbon and alloying elements in the steel composition.
The temperature at the end of martensitic transformations is designated Mk. In the temperature range between Mn and Mk, a diffuse-free restructuring of the steel crystal lattice occurs. When the temperature Mk is reached, diffuseless recrystallization stops. For high-carbon alloy steels it can be negative.
Practice of heat treatment of steels for martensite
In large-scale and mass production, automatic conveyor lines are used for hardening steel products, which carry out a full cycle of obtaining the necessary martensitic structure for certain grades of steel.
In tool shops and pilot plants, tools and parts are hardened manually by heating the tool in muffle furnaces, in baths with oils, salts or molten metals. Cooling is carried out in different media: water, oil, air. The temperature and hardening process parameters are developed by the technologist in accordance with technical standards and steel grades.
Surface heating is carried out in cases where it is necessary to increase the strength of the outer layers of products while maintaining a soft core. Surface hardening is carried out in high frequency generators.
Depending on the required heating temperature, various salts or mixtures of salts are used; So, for high-temperature heating (1000-1300 degrees), molten barium chloride is used; for heating up to 750-950 degrees, mixtures of salts of barium chloride, potassium chloride and sodium chloride are used. For low-temperature heating of 300-550 degrees, mixtures of potassium and sodium nitrate are used.
Liquid media of varying cooling abilities are most often used as cooling media for martensite quenching. Usually water is used, and the rate of heat removal is increased by adding caustic soda. Softer coolants include mineral and transformer oils.
Types of hardening for martensite
- Continuous, or hardening in one environment.
- Hardening in two environments.
- Step hardening.
- Cold treatment.
After heating the steel product to the temperature of the austenite fraction, it is sharply cooled either in water (the simplest option), or in heated oils, or in air, depending on the composition of the steel. With this cooling method, warping and sometimes cracks appear.
To avoid risks, hardening is used in two environments. After heating, the product is immersed in water, kept for some time, and then further cooling to a temperature of Mk occurs in a softer environment. This method is suitable for mass production.
With a stepwise cooling scheme, the steel is immersed in a coolant with a temperature exceeding MP by 60-100 degrees, maintained for the estimated time, and then cooled in still air. Small-sized tools made of medium- and low-alloy steels are subjected to this type of cooling.
Cooling in cold (liquid nitrogen) is usually resorted to in cases where Mk for a steel grade is below zero. These are high-alloy carbon grades used for the manufacture of measuring tools and elements of rolling bearings.
Hardening of steels for martensite
The purpose of quenching and tempering (two-stage heat treatment) is to provide a set of necessary mechanical properties, in particular increased strength compared to annealing and normalization.
Quenching and tempering is used for a wide range of carbon and alloy engineering and tool steels. Phase transformations in these steels correspond to the iron-cementite or iron-carbon-alloying element phase diagrams.
Hardening of steels
(alloys based on a polymorphic metal) is a heat treatment operation that consists of heating into a single-phase austenitic region (sometimes into two-phase regions) and rapid cooling at a rate that prevents the equilibrium decomposition of austenite (eutectoid transformation).
Quenching is carried out to obtain martensite as an independent phase or in combination with austenite, troostite or cementite. This structural-phase state is considered as a preparatory stage for subsequent tempering.
Technological parameters during hardening for martensite are heating temperature and cooling rate. The hardening temperature is chosen in relation to the critical points (lines) on the phase diagram - Ac1, Ac3, Ast. Hardening from temperatures above Ac3 and Ast is called full hardening
, and hardening from temperatures in the range Ac1—Ac3 or Ac1—Ast is called
incomplete hardening
. Cooling during quenching must be continuous, intense, at a rate above critical (Fig. 13.9).
The critical cooling rate
of steels (Vcr) is the minimum cooling rate at which austenite transforms into martensite.
Full hardening is applied to hypoeutectoid steels, i.e. from the austenitic region, from temperatures above Ac3 by 30...50 °C. For hypereutectoid steels, incomplete hardening is applied, i.e. from the austenite + cementite region, from temperatures 30...50 °C above Ac1 (Fig. 13.10).
In carbon steels, the nonequilibrium martensite phase obtained as a result of quenching is:
— interstitial solid solution;
— interstitial solid solution, supersaturated with respect to the equilibrium carbon content (0.006%) in ferrite at room temperature.
Carbon martensite has a body-centered tetragonal (bct) lattice, which differs significantly from the original fcc lattice of austenite. The tetragonality of the lattice (the ratio of the c/a axes) increases with increasing carbon content.
The microstructure of martensite is complex: it is either packet or lath martensite. The crystal morphology depends on the transformation intervals. If the transformation range is above room temperature (up to 100 °C), then martensite crystals take on the shape of laths (Fig. 13.11, b). In high-carbon steels with a reduced transformation range, the crystals are plates (Fig. 13.11, a; 13.12).
A significant difference in the specific volumes of austenite and martensite of carbon steels (about 3%) causes not only plastic deformation inside the grains of the solid solution, but also elastic deformation in the volume of the product. Added to this are thermal stresses. As a result of sharp cooling during quenching, workpieces with a martensitic structure are prone to warping (“warping”) - an uneven change in shape and size, which is greater the more complex the shape of the part. This is the main disadvantage of hardening carbon steels.
In a number of carbon and alloy steels, during quenching from the austenitic region, a bainitic transformation may occur (see Fig. 13.8, region A -> B). The two-phase structure ferrite + cementite (a + C) obtained as a result of such a transformation is not called pearlite, because it was not formed during the eutectoid transformation. In addition, the shape of the carbide particles is not plate-like; the particles themselves are highly dispersed.
Based on the dispersion, arrangement of carbides and the structure of the a-phase, upper and lower runes are distinguished. Lower bainite has the best properties: it is formed at temperatures below 350 °C; dispersed carbide particles are located inside ferrite grains. This structure provides a combination of high strength, ductility and toughness.
Isothermal hardening based on bainite transformation is widely used for products made of alloy steels, providing them with high structural strength.
One of the main technological properties of steel during hardening is hardenability
— the ability of steel to acquire, as a result of hardening, a martensitic or martensitic-troostite structure with high hardness in a layer of a certain size. Hardenability is quantitatively characterized by the critical diameter dcr.
Critical diameter
- the maximum diameter of the workpiece at which, as a result of hardening, a martensitic or semi-martensitic (50% martensite + 50% troostite) structure is obtained.
The critical diameter is inversely proportional to the critical cooling rate:
dcr = f(1/Vcr).
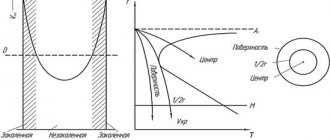
Hardenability is determined by the rate at which temperature during rapid cooling spreads across a cross-section of a workpiece of a specific size with a specific thermal conductivity. At a high cooling rate, it is possible to ensure martensitic transformation in the workpiece only if its size is small. Cooling at a critical rate of large products only takes place in the near-surface layers. In the deep layers, cooling occurs at a rate less than the critical one, which leads to the equilibrium decomposition of austenite with the formation of pearlite. Due to the difference in cooling rates across the cross section of the product, there is also a difference in the phase composition: in the near-surface layer there is martensite, and in the center there is ferrite + cementite (Fig. 13.13).
In order to thoroughly anneal large-section workpieces, they are made from alloy steels, since all alloying elements (except cobalt) reduce the critical hardening rate.
The choice of one or another cooling medium (water, oil, air, polymer media) during volumetric hardening is determined by the critical cooling rate. If alloy steels with a reduced critical hardening rate are used, then “softer” media can be used - oil or air instead of water.
Based on the volume of the product to which hardening is applied, a distinction is made between volumetric and surface hardening. Most products are subjected to volumetric hardening, when a martensitic (martensitic-troostite) structure is obtained over the entire cross-section.
Surface hardening is used for parts in which, due to operating conditions, high surface hardness, wear resistance, and also a high endurance limit are required.
To reduce the high level of residual elastic stresses, which are characteristic of hardened steel parts, stepwise cooling is also carried out during volumetric hardening (Fig. 13.14, a).
Thermal stresses are reduced under the condition of uniform heating before the martensitic transformation, which is achieved using step-by-step hardening: the workpiece, heated to the hardening temperature, is transferred to a liquid medium with a temperature 50...100 °C higher than the temperature at which the martensitic transformation begins, and a soak is made to equalize the temperature across the cross section and cooled in air.
During
isothermal hardening,
steel does not undergo martensitic transformation. The exposure is carried out at a temperature when not a martensitic, but a bainitic phase transformation occurs (Fig. 13.14, b). In the process of isothermal hardening, structural stresses are almost completely eliminated. However, the bainite transformation does not occur in all steels.
Surface hardening
steels are made in order to increase the hardness, wear resistance of the surface and the endurance limit of parts (gear teeth, shaft journals, guide frames of metal-cutting machines, etc.). The core of the part remains viscous and absorbs shock and other loads well. For surface hardening, various surface heating methods can be used: gas flame, high frequency currents, electric contact, laser, electron beam, plasma, etc.
For products of simple shapes, induction hardening with heating by high-frequency currents
(HDTV). With this method, the product is placed in an alternating electromagnetic field created by an inductor - a single or multi-turn copper pipe circuit through which an alternating electric current is passed. Heating occurs as a result of the fact that eddy currents (Foucault currents) are induced near the surface of the product. Cooling during hardening is carried out through the holes of the inductor (after turning off the current) using a water sprayer or water-air method.
Advantages of high-frequency hardening compared to volumetric hardening:
• formation of finer grains;
• increase in 2-3 times the endurance limit;
• reduction of heat treatment time, and therefore increased productivity;
• obtaining products without scale;
• reduction of warping (involuntary change in shape) during hardening;
• the possibility of complete mechanization and automation of the process (incorporating it into the processing production line without interrupting the technological cycle).
Two types of martensite
Martensitic steel can contain one of two types of martensite:
- rack and pinion;
- lamellar.
Lath martensite is also called dislocation martensite. It is formed in steels with low and medium carbon content. Lath martensite also forms in steels with a high alloy content. Martensitic transformation in such alloys begins only at temperatures above 300 degrees.
In lamellar martensite, transformation begins at temperatures below 200 degrees. Twin or lamellar martensite forms in high carbon alloy steels.
Types of steel tempering
The main technical parameter of the OS is the heating temperature. There are 3 types of OS - high, medium and low. Of course, high-temperature tempering is the optimal means of processing, since the higher the heating temperature, the more actively recrystallization of the metal will occur. However, low- and medium-temperature processing methods also have practical benefits that should not be underestimated. Below we will look at each type of OS separately.
High
High tempering of steel is a variant of tempering treatment at temperatures from 500 to 700 degrees. This method is the most effective, since with such heating, polygonization and recrystallization of the material occurs, which eliminates all stress inside the metal. Usually lasts from 2 to 3 hours. In the case of processing complex structures, the recommended time can increase to 6 hours.
The main disadvantage of high-temperature tempering is a slight decrease in the strength of the material. Therefore, the technique is not suitable for processing parts that will experience extremely high loads during operation. The high temperature technique applies to all types of steel, but note that in the case of some alloy alloys, so-called reversible high temperature embrittlement may occur during processing.
Short
Low tempering of steel is a method of processing a steel alloy or product in which heating is carried out to a temperature of 100 to 250 degrees. The processing time is usually 1-3 hours depending on the type of part and its dimensions. During low-temperature processing, diffusion of particles of carbon components occurs without polygonization and recrystallization of the atomic lattice. This allows you to increase some of the physical characteristics of the material - strength, ductility, hardness, chemical inertness.
Low tempering is a universal technology, but in fact it is used mainly for tempering products made from low-alloy and high-carbon steels (knives, utensils, simple parts). You also need to avoid heating the material above a temperature of 250 degrees (otherwise it will fall into a type I island of fragility, which can lead to irreversible damage to the metal).
Average
The main feature of medium tempering is the active diffusion of carbon without polygonization and recrystallization of the alloy. In the case of medium-temperature treatment, the elasticity of the material improves and its relaxation resistance increases. The steel tempering temperature in this case ranges from 350 to 500 degrees. The average processing time is 2-4 hours. The optimal environment is oily or alkaline. Medium processing is well suited for durable parts of complex shapes - springs, springs, impact structures. However, in practice this technology is rarely used due to a number of limitations:
- In the temperature range from 250 to 300 degrees there is a so-called island of brittleness of the first kind, which should be avoided. At the same time, at temperatures above 500 degrees, there is another island of fragility of the second type (it is also recommended to avoid it). We will discuss the features of these islands below. A slight deviation in temperature up or down during the holidays can lead to fatal consequences.
- The technique has no advantages in comparison with alternative technologies (low and high). At the same time, weak processing furnaces usually cannot heat the working environment to such temperatures, and stronger furnaces can reach higher temperatures, which is inconvenient from a practical point of view.
This is interesting: Corrosion-resistant steel - choosing quality grades
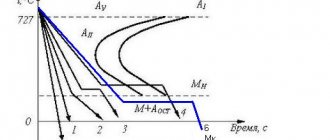
26.Transformation in hardened steel during heating (tempering): three stages of tempering.
Tempering is the operation of heating hardened steel to reduce residual stresses and impart a set of mechanical properties that are necessary for long-term operation of the product. Tempering is carried out by heating parts hardened to martensite to a temperature below critical. In this case, depending on the heating temperature, the states of martensite, troostite or tempered sorbitol can be obtained. These states differ from the hardening states in structure and properties: during hardening, cementite (in troostite and sorbite) is obtained in the form of elongated plates, as in lamellar perlite. And when tempered, it turns out granular or dotted, as in granular perlite.
When tempering steel hardened to martensite, transformations occur in it, which lead to the decomposition of martensite and the formation of an equilibrium structural-phase composition. The intensity and result of these transformations depend on the tempering temperature. The tempering temperature is selected depending on the functional operational purpose of the product.
Depending on the heating temperature, there are three types of tempering: low-temperature (low), medium-temperature (medium) and high-temperature (high).
During low tempering (heating to a temperature of 200–300°), martensite mainly remains in the steel structure; in addition, iron carbides begin to separate from the solid solution of carbon in b-iron and their initial accumulation in small groups begins. This entails a slight decrease in hardness and an increase in the plastic and viscous properties of steel, as well as a decrease in internal stresses in parts.
Medium temperature (medium) tempering is carried out at temperatures from 350 to 450 °C. With such heating, the decomposition of martensite is completed, leading to the formation of ferrite and cementite that are normal in composition and internal structure. Due to the insufficient intensity of diffusion processes, the grain size of the resulting phases turns out to be very small.
High temperature (high) tempering is carried out at 500–650 °C. Under such heating conditions, with increased diffusion processes, the formation of larger ferrite and cementite grains occurs, accompanied by a decrease in dislocation density and the complete elimination of residual stresses.
The martensite decomposition product obtained during high tempering, called tempering sorbitol, has the maximum viscosity for steel.
This complex is ideal for machine parts subjected to dynamic loads. Due to this advantage, heat treatment, combining hardening and high tempering, has long been called an improvement.
What is temper brittleness
The release temperature affects the quality of processing - the higher the temperature, the higher the quality of processing. However, metallurgical scientists have found that this rule has 2 exceptions, when an increase in temperature does not lead to an improvement, but to a deterioration in the quality of the material. These two exceptions are often called temper brittleness islands in practice. Fortunately, several effective, safe ways to bypass these islands have been developed, so the issue of tempering ability is not significant in modern metallurgy. Let's look at each of the islands separately + learn how to get around them.
Is it possible to temper steel at home?
Most often, heat treatment applies to various simple parts, household utensils - knives, forks, metal cups, car parts, and so on. However, home metallurgy has many limitations that the common man may not be aware of. Let's consider the main problems that a person may encounter during a steel holiday at home:
- Most home ovens cannot reach high temperatures. Therefore, at home you can only make a low or medium vacation. Theoretically, you can try to re-equip or “strengthen” your stove to increase the heating temperature, but this will be difficult for a person without experience.
- To carry out heat treatment, it is necessary to use a protective medium (oil, alkali, saltpeter). But each substance has its own temperature characteristics. A simple example: saltpeter-based compounds can explode when heated to high temperatures, which can be dangerous to the life and health of the home metallurgist.
- Tempering without the use of a protective environment can be fatal to the metal itself. The fact is that without the use of a protective environment, the metal will cool quickly, which can affect the quality of the steel (increased brittleness, bending, plastic deformation, rust).
- Also, do not forget about low-temperature brittleness of the first type (from 250 to 300 degrees). If the temperature is incorrect, the quality of the metal can be seriously affected, up to the complete destruction of the alloy.
Tempered martensite
When martensite is removed from the quench bath, it is called freshly quenched martensite. The hardness data in Figure 4 refers specifically to freshly quenched martensite. The big problem with this "fresh" martensite is that if the carbon content is more than 0.2-0.3%, then the steel in this state is very brittle. This brittleness can be eliminated by some loss of hardness if the hardened steel is slightly heated. This process is called vacationing.
Therefore, hardened steels are almost always tempered to increase the toughness of the steel. The resulting martensite is called tempered martensite. The increased tempering temperature allows the carbon atoms that are “trapped” in the bct structure to move a little. This movement of atoms gives two effects: – allows the bct structure to change into a bcc structure; – enables the formation of very small carbide particles.
Transformation of austenite to martensite at high cooling rates
This transformation occurs at high cooling rates, when diffusion processes are suppressed.
When steel is cooled at a rate greater than the critical one (V > Vk), the transformation begins at the temperature of the beginning of the martensitic transformation (Mn) and ends at the temperature of the end of the martensitic transformation (Mk). As a result of this transformation of austenite, a hardening product—martensite—is formed.
The minimum cooling rate Vk, at which all austenite is supercooled to a temperature of t.Mn and transformed, is called the critical quenching rate.
Since the diffusion process does not occur, all the austenite carbon remains in the lattice and is located either at the centers of tetrahedra or in the middle of long ribs.
During the formation of martensite, the cubic lattice is greatly distorted, turning into a tetragonal one. Lattice distortion is characterized by the degree of tetragonality: c/a > 1. The degree of tetragonality is directly proportional to the carbon content in the steel.
Crystal lattice of martensite
The mechanism of martensitic transformation has a number of features.
1. Non-diffusion nature.
The transformation occurs via a shear mechanism. At the beginning of the transformation, there is a continuous transition from the austenite lattice to the martensite lattice (coherent bond). When a face-centered cubic lattice transforms into a body-centered cubic lattice, the atoms are displaced to distances less than interatomic ones, i.e. there is no need for self-diffusion of iron atoms.
2. Orientation of martensite crystals.
The crystals have the shape of plates, tapering towards the end; under a microscope, this structure looks like a needle. The resulting plates instantly grow either to the austenite grain boundary or to a defect. The next plates are located to the first at angles of 60 o or 120 o, their sizes are limited by the areas between the first plates.
The oriented (coherent) growth of martensite crystals provides minimal surface energy. During coherent growth, due to the difference in the volumes of austenite and martensite, large stresses arise. When martensite crystals reach a certain size, these stresses become equal to the yield strength of austenite. As a result, coherence is disrupted and the martensite lattice is separated from the austenite lattice. Crystal growth stops.
3. Very high crystal growth speed, up to 1000 m/s.
4. Martensitic transformation occurs only with continuous cooling. Each steel begins and ends at a specific temperature, regardless of the cooling rate. The temperature at which the martensitic transformation begins is called the martensitic point MN, and the temperature at the end of the transformation is called MC. The temperatures MH and MC depend on the carbon content and do not depend on the cooling rate. For steels with a carbon content above 0.6%, the MC goes into the region of negative temperatures.
The influence of hardening on the characteristics of austenite decomposition. Martensite
Quenching is a type of heat treatment, the essence of which is rapid heating to high temperatures above the critical points Ac3 and Acm, followed by rapid cooling. If the temperature decreases with the help of water at a rate of more than 200˚C per second, then a solid needle-shaped phase called martensite is formed.
It is a supersaturated solid solution of carbon penetration into iron with an α-type crystal lattice. Due to powerful movements of atoms, it is distorted and forms a tetragonal lattice, which is the cause of hardening. The formed structure has a larger volume. As a result of this, crystals confined to a plane are compressed, and needle-shaped plates are born.
Martensite is durable and very hard (700-750 HB). Formed exclusively as a result of high-speed hardening.
Martensite
| Three types of planes of the most dense packing in martensite with a periodic layered structure formed from the initial /32 phase of the CsCI type. The arrow denotes the displacement vector of each layer relative to layer A, taken as the origin.| Six types of layers of the most dense packing in martensite with a periodic layered structure formed from the initial ftt phase of the Fe3A type. |
Martensite with a 3H or 9R structure, consisting of three close-packed planes A, B and C, is formed in (32-alloys with an initial phase of the CsCI type. However, martensite with a 2H structure is found in all alloys.
Martensite, which for steels is supersaturated solid solution of carbon in a-iron, decomposes under the influence of temperature - carbon is released from the lattice of o-iron.
Martensite is a structural component of crystalline solids resulting from the martensitic transformation.
| Schemes of the main types of heat treatment of steels. |
Martensite is obtained by implementing only the first stage of secondary crystallization and has a characteristic plate-like structure, which under a microscope is needle-shaped. The growth of plates by shear occurs instantly at a speed of about 1000 m/s according to a diffusion-free mechanism, since the diffusion transition of atoms from austenite crystals to martensite at low temperatures is impossible.
Martensite has the largest specific volume compared to other structural components of steels and especially austenite. An increase in the specific volume during the formation of martensite leads to the occurrence of large internal stresses during quenching, causing deformation of products or even the appearance of cracks.
| The unit cell of martensite (a. martensite. |
Martensite is a very hard and durable structure. It is harder and stronger than bainite. But its plastic properties are low, especially impact strength. Martensite contains high residual stresses that arose as a result of an increase in specific volume as a result of transformations and are not eliminated due to the low plasticity of martensite.
Martensite, which after quenching has a crystal lattice with a tetragonal unit cell, begins to transform into cubic when heated above 80 C. Like any supersaturated solution, martensite is unstable. It decays at room temperature, but the decay rate is extremely low due to the low thermal mobility of the atoms. At temperatures above 80 C, the mobility of atoms is sufficient for carbon to partially transfer from a supersaturated solution into carbide plates with a thickness of only a few atomic layers in a relatively short period of time. This transformation occurs in the range from 80 to 170 C and is accompanied by a decrease in the distortion of the martensite crystal lattice. Internal stresses are reduced, the specific volume of martensite decreases, and the dimensions of the part are slightly reduced. Hardness and strength remain unchanged, but plastic properties increase slightly.
| Tetragonal cell of the a-phase in the crystal lattice of the austenent. |
Martensite in steel has a tetragonal lattice, apparently, even at a low carbon content (0-1%), if it is formed under conditions under which diffusion processes practically do not occur. But in low-carbon martensite (0 5% C), as a result of diffusion processes, the tetragonal lattice can transform into a lattice with cubic symmetry.
Martensite without internal twins has been observed in low-carbon steel (where these twins are usually adjacent to hexagonal e-martensite) and in manganese and chromium steel.
| Two methods of heating for hardening. |
During quenching, martensite is obtained only if it is cooled at a rate exceeding a certain, so-called critical rate. Each grade of steel has its own critical speed.
Bottom line
Fundamental research into martensitic transformations, begun by the Soviet scientist G.V. Kurdyumov, who first proposed the theory of diffuse-free martensitic transformation, has been going on for more than 60 years. Technologies based on the “unusual physical and mechanical properties” of martensitic materials may be especially in demand in the most advanced industries. In defense, aerospace, precision instrumentation, electronics, nanoproduction, medicine and even cosmetology.
Sources
- https://ArmRinok.ru/stal/martensitnoe-prevrashchenie.html
- https://intehstroy-spb.ru/spravochnik/martensit-i-martensitnye-stali-struktura-kristallicheskaya-reshetka-svoystva.html
- https://prompriem.ru/stati/martensit.html
- https://martensit.ru/stal/martensit/
- https://met-all.org/stal/martensit-martensitnoe-prevrashhenie-stali.html
- https://lux-stahl.ru/raboty/martensit-otpuska-struktura.html
- https://PlazmoSvarka.ru/metally/martensit-eto-materialovedenie.html
- https://www.abt-group.su/articles/martensitnye-stali-i-ih-osobennosti
- https://melt-spb.ru/raboty/martensitnaya-struktura.html
- https://msmetall.ru/metalloobrabotka/struktura-martensita.html