Based on the characteristics of the marking of carbon steels, an alloy with the name U10 should contain about 1% carbon (0.96-1.03%). What effect does such an amount of this element have on the material? Tool carbon steel grade U10 is characterized by low heat resistance and relatively low hardenability. As a result, the alloy is not used for welding work, as well as for casting large-sized elements. Most often, cutters that operate at low speeds are made from this metal. This is due to a noticeable drop in the hardness of the U10 alloy at t>190–200°C.
Characteristics of U10 steel
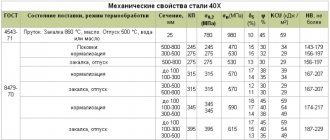
With a specific gravity of 7810 kg/m3 and hardness HB 10-1 = 197 MPa, steel U 10 can be comfortably processed by cutting and forging: K υ tv. spl=1.1, Кυ b.st=1.0 and t=1180-800оС. The material is not prone to temper brittleness and is non-flock sensitive. The remaining physical and mechanical properties of the U10 carbon alloy are presented in the tables:
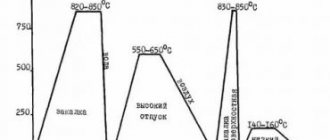
Stages of the steel normalization process
Normalization annealing is carried out in several stages. All operations are carried out at metallurgical and metalworking enterprises equipped with thermal furnaces of various designs and other specialized equipment.
Heat
Hypoeutectoid steels, during normalization, are heated to temperatures above the Ac3 point by 40...50 °C. For hypereutectoid steels, a lower heating temperature is selected, which makes it possible to eliminate the growth of austenitic grains and the formation of a coarse mesh during normalization. The specific temperature depends on the carbon content and alloying elements, if any.
Most often, isothermal and thermokinetic diagrams are used to determine the optimal temperature regime. For newly developed steel grades, the theoretically calculated values are confirmed experimentally. The period of phase transformations is determined by the nomenclature and quantity of alloying components. For unalloyed and low-alloy steels, 1.5 minutes of heating is usually set for each millimeter of product thickness.
Excerpt
Holding time is the time that the product must remain in the heating chamber at a given temperature. This stage is necessary for complete and uniform heating of the charge and completion of phase transformations. The holding time depends on the steel grade, product dimensions, and heating temperature. For small parts of simple configurations, to heat up the entire volume, it is enough to hold the metal product at a given temperature for 15 minutes.
Cooling
Cooling is carried out mainly in still air. Air blowing is sometimes used. With accelerated cooling, austenite decomposes at low temperatures, which ensures the appearance of a dispersed ferrite-cementite structure. The structure of normalized medium- and high-carbon steels is characterized by higher strength and hardness compared to the annealed state. The environment and therefore the cooling rate significantly influence the structure and other characteristics. One steel, heated to the same temperature, but cooled under different conditions, has different characteristics.
In rolling shops, normalization according to the scheme described above can be replaced by normalization rolling. This operation is carried out on a rolling mill using heating heat, to which the rolled metal is subjected before rolling. This technology makes it possible to obtain a rolled steel structure similar to the normalized state (fine-grained, with uniform mechanical characteristics throughout the entire volume), but at much lower energy and labor costs.
Steel U10: application
U10 steel is supplied to the workshops of industrial enterprises in the form of rolled products in accordance with approved GOSTs:
- GOST 21997-76 and GOST 2283-79 – tapes
- GOSTs 7417-75, 8559-75, 8560-78 and 1435-99 – calibrated rods
- GOST 14955-77 and 1435-99 – ground rods and silver
- GOST 1133-71, 4405-75 and 1435-99 – forgings
- GOST 4405-75 and 103-2006 – stripes
The main scope of application of the U10 alloy is cutters, saws - tools designed for wood processing. In addition, carbon tool steel U10 allows you to make high-quality needle wire, various cold stamping parts, coiled springs and other spring parts, taps and dies, simple gauges, as well as working parts of hand-held metalwork tools: files, scrapers, etc.
Specifications
The main characteristic of ShKh15 steel is its high sensitivity to technological processing by hot deformation and thermal processes. As a result, the steel gains durability, which directly affects the quality of the finished products. These processes form high wear resistance and elasticity while maintaining the required level of viscosity and plasticity.
Quenching is carried out in an aqueous solution at a temperature of 810–820 or in oil, which is heated to 40–60C. The temperature of the workpiece should be from 650 to 830C
The main advantages of ShKh15 steel are the following:
- uniformity achieved through the use of special technologies;
- excellent endurance in contact with other materials;
- amenability to processing;
- high hardness and wear resistance;
- viscosity and plasticity;
- obtaining a thin, sharp cutting edge.
The disadvantages of Shx15 steel usually include instability to corrosion processes and the difficulty of sharpening.
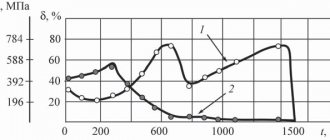
The influence of heat treatment on the hardness of steels 45 and U10
| Heat treatment mode | Heating temperature, 0C | Heating duration, min | Exposure time, min | Cooling medium | H.R.C. | HB |
| Steel 45 | ||||||
| Annealing Normalization Quenching Tempering Tempering Tempering | ||||||
| Steel U10 | ||||||
| Annealing Normalization Quenching Tempering Tempering Tempering |
Table 7.3
Effect of carbon content on hardness of hardened
become
| steel grade | Carbon content, % | Hardness |
| HRB | H.R.C. | HB |
| U8 U12 | 0,2 0,45 0,8 1,2 |
Contents of the report
1. Topic and purpose of the work.
2. Brief answers to security questions.
3. Area of the state diagram of alloys of the Fe – C , relating to steels with temperature ranges for heating steels for heat treatment.
4. Modes of annealing, normalization, hardening and tempering of steels 45 and U10.
5. Results of measuring the hardness of steels 45 and U8 after various types of heat treatment in accordance with the specifications.
6. Conclusions.
Laboratory work No. 8
STRUCTURE OF STEEL IN A NONE-EQUILIBRIUM STATE
Purpose of the work : to study the effect of quenching and tempering on the structure of carbon steels, to establish a connection between the structure of heat-treated steels, their diagrams of isothermal decomposition of austenite and mechanical properties.
THEORETICAL INFORMATION
The performance properties of steel depend on its chemical composition and structure. The desired change in structure, and, consequently, mechanical properties, is achieved by heat treatment. Various structures of steel are formed during its cooling from the austenitic state.
A slight degree of supercooling or very slow cooling ensures that equilibrium structures are obtained (laboratory work No. 7). The greater the degree of overcooling of austenite or the rate of its cooling, the lower the temperatures at which the transformation of austenite occurs, the more nonequilibrium the structure of the resulting steel. In this case, steel can acquire the structures of sorbitol, troostite, acicular troostite (bainite) or martensite.
Hardening, which ensures the production of the most nonequilibrium steel structure - martensite, is accompanied by the occurrence of large internal stresses. Since these stresses can cause warping or failure of the part, they are reduced by tempering.
Rice. 8.1. Microstructure of hardened low-carbon (0.15% C) steel. X200
When tempering, tempering structures (troostite, sorbitol, pearlite) are formed from the structures of hardened steel. Let us take a closer look at the structures of carbon steels formed during hardening and then during tempering. The resulting steel structure depends not only on the cooling rate of the austenite, but also on the heating temperature and chemical composition of the steel.
Low-carbon steel, containing up to 0.15% carbon, heated above the AC3 temperature and quenched in water, has the structure of low-carbon martensite (Fig. 8.1).
Rice. 8.2. Change in the temperature range of martensitic transformation - a
(region
Mn - Mk is shaded, solid line - troom ) and the mass fraction of retained austenite - b
(possible share
Aost , shaded) from the carbon content in steel
Martensite -
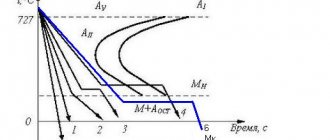
it is a supersaturated solid solution of carbon in a-iron. It contains as much carbon as was in austenite, i.e. in steel. Martensite has a tetragonal body-centered lattice. With increasing carbon content, the tetragonality of the martensite crystal lattice and the hardness and strength of hardened steel increase. It has a characteristic lamellar, needle-like structure under a microscope. The growth of martensite plates occurs at a speed of about 1000 m/s according to a diffusion-free mechanism. They are oriented with respect to each other at an angle of 60 and 120° in accordance with certain crystallographic planes of austenite within the austenite grain, and the higher the heating temperature for hardening and the, therefore, the larger the austenite grain, the more coarsely needle-shaped and brittle it will be.
The hardness of martensite is very high, for example, for medium-carbon steel - 55...65 HRC, (HB = 5500...6500 MPa). The transformation of austenite into martensite is accompanied by an increase in the specific volume of steel, since martensite has a larger volume than austenite. In steels containing more than 0.5% C, the complete transformation of austenite into martensite does not occur and the so-called retained austenite remains. The higher the carbon content in steel, the lower the temperature range ( Mn–Mk ) of the martensitic transformation (Fig. 8.2, a
) and more retained austenite (Fig. 8.2, b).
When treated with cold, it is possible to reach a temperature Mk and ensure the transition of residual austenite to martensite.
In hypoeutectoid steels, quenched at optimal temperatures (30...50 °C above AC3 ), martensite has a finely needle-like structure (Fig. 8.3).
Hypereutectoid steels are subjected to incomplete hardening (heating temperature is 30...50 0C higher than AC1 ). The steel acquires a martensite structure with evenly distributed grains of secondary cementite and retained austenite (5...10% Arest .) (Fig. 8.4).
After complete hardening, hypereutectoid steel has the structure of coarse-needle martensite and contains over 20% retained austenite (Fig. 8.5). Such steel has significantly lower hardness than after incomplete hardening.
Rice. 8.3. Hardening martensite in hypoeutectoid steel. X600
Rice. 8.4. Microstructure of hardened hypereutectoid steel:
martensite, residual austenite, secondary cementite grains. X400
Rice. 8.5. Microstructure of overheated hardened steel:
coarse-needle martensite, residual austenite. X400
Rice. 8.6. Microstructure of quenched troostite:
A -
magnification 500; b – magnification 7500
Martensite hardening is achieved by cooling carbon steels in water at a speed above critical. When steel is cooled more slowly from the austenitic state, for example, in oil at a rate less than critical, austenite at temperatures of 400...500 oC decomposes into a highly dispersed ferrite-cementite mixture of lamellar structure, called quenching troostite .
Troostite is a structure with increased etchability (Fig. 8.6, a) and a characteristic lamellar structure (Fig. 8.6, b).
Even slower cooling of steel (for example, in a stream of cold air) causes, at temperatures of 500...650 0C, the decomposition of austenite into a ferrite-cementite mixture, which is coarser than troostite, also of a lamellar structure, called quenching sorbitol. As the cooling rate decreases and the transition from martensite structures to troostite, sorbitol and, finally, pearlite, the hardness of the steel decreases.
Rice. 8.7. Microstructure of troostite (a) and sorbitol (b) tempered. X7500
When heated, steel with a nonequilibrium martensitic structure acquires an equilibrium pearlite structure. When hardened steel is heated to temperatures of 150...250 °C (low tempering), a structure of cubic (tempered) martensite .
An increase in tempering temperature (300...400 °C - medium tempering and 550...650 °C - high tempering) leads to the appearance of a structure of granular
troostite and tempering sorbitol
, respectively. These structures are shown in Fig. 8.7, a and 8.7, b. Steel with a troostite structure with a hardness of 35...45 HRC (HB = 3500...4500 MPa) provides maximum elasticity, which is usually necessary in the manufacture of springs, springs, and membranes. Steel with a tempered granular sorbitol structure (25…35 HRC) has the best complex of mechanical properties and high structural strength. That is why hardening and high tempering are called thermal improvement.
Heating of hardened steel up to temperature AC1 (727 °C) ensures that an equilibrium structure of granular pearlite is obtained, i.e. less dispersed than sorbitol and troostite, ferrite-cementite mixture. If the steel is hypoeutectoid, grains of excess ferrite are separated in it.
Thus, when austenite is overcooled as the cooling rate increases, pearlite, sorbite, troostite of a lamellar structure and quenched martensite are formed, and when martensite decomposes as the tempering temperature increases, cubic (tempered) martensite, troostite, sorbite, and pearlite of a granular structure are formed.
Granular structures formed during tempering are characterized by higher ductility and impact strength compared to similar structures with a lamellar structure.
Work order
1. Familiarize yourself with theoretical information and, if necessary, determined by the teacher, take a theoretical test on the topic.
2. Draw a double diagram of the state of iron-carbon alloys, its section corresponding to steels and plot on it the temperature ranges for heating steels for heat treatment.
3. Draw diagrams of isothermal decomposition of austenite for the steels under study and plot heat treatment modes on them (isothermal holding temperatures, cooling rates).
4. Study and sketch the microstructures of heat-treated steels, indicate their hardness.
5. Draw conclusions and report on the work in accordance with the assignments.
Control questions
1. What is martensite called? What are its structure and properties?
2. Which phase is called retained austenite? What causes retained austenite to appear in hardened steel? Conditions on which the amount of retained austenite in the structure of hardened steels depends? The influence of retained austenite on the properties of hardened steels.
3. Optimal heating temperatures for hardening hypoeutectoid and hypereutectoid steels. What are the structure and properties of steels after hardening?
4. What is called sorbitol, hardening troostite, tempering sorbitol and tempering troostite? Conditions for the formation of these structures. What are their structure and properties?
5. What is called low, medium and high vacation?
Contents of the report
1. Topic and purpose of the work.
2. Brief answers to security questions.
3. Area of the state diagram of alloys of the Fe – C , relating to steels with temperature ranges for heating steels for heat treatment.
4. Diagrams of isothermal decomposition of austenite for the steels under study with heat treatment modes (isothermal holding temperatures, cooling rates).
5. Results of microstructural analysis of alloys performed in accordance with the assignments.
6. Conclusions.
Laboratory work No. 9
Advantages of U10 steel
The positive properties of the U10 series knives make up an extensive list.
- Spicy . Excellent cutting qualities. The hardness of knives made of tool alloy steel is checked according to GOST 9012 with the decarbonized layer removed at a distance of 10 cm from the strip. The number of prints should not exceed three.
- Maintains sharpness for a long time . The cutting edge of the product remains sharp for a long time. Knives made of U10 steel easily pass tests with hard, soft and abrasive materials, showing competitive advantages when cutting material at various angles.
- Abrasion resistance . Knives of the U10 series are resistant to chipping (bending edges) when cutting small hunting trophies, used at home and while fishing. However, manufacturers of products made from hard steel alloys warn about possible damage to the structure of the metal when carrying out complex tasks: Cutting hard workpieces - wood, nails, metal structures, cattle bones.
- Opening cans.
- Replacement of hammers, screwdrivers, similar tools.
- Work with aggressive environments.
- Bend at a large angle.