Residual pressure in cylinders - article GasZaChas
Home » Articles » Residual pressure in cylinders
When operating cylinders, it is prohibited to completely remove (consume) the technical gas contained in them due to safety precautions.
There should be residual pressure in the container. But how necessary is this, and what does this residual pressure produce? The residual pressure in the cylinder must be at least half an atmosphere (0.05 MPa). This amount is enough to, if necessary, check the stream on a gas analyzer and find out what gas was contained in the cylinder. This will avoid mixing flammable gases with oxygen, because even small residues of methane, propane, acetylene, and oil can cause an explosion. Agree, to avoid such a gloomy prospect, it’s no sin to play it safe.
Of course, such risks are minimized, because any more or less knowledgeable person will say that it is almost impossible to confuse cylinders for flammable and non-flammable gases. It's like not being able to tell a hippopotamus from an elephant. However, human resourcefulness knows no bounds, and cases where cylinders were used for gases other than those intended for them are known. In addition, residual pressure is a guarantee that the cylinder has not been filled with foreign liquids and substances (for example, air, oil, water) at a time when it was not used for its intended purpose. Many people don’t know, but when regular motor oil interacts with oxygen, an explosion can occur. Oxygen cylinders without residual pressure are not accepted for refilling, because Even a drop of oil entering the container can cause an explosion!
Is it possible to “reanimate” a gas cylinder without residual pressure and make it suitable for use? This is possible, but in this case a thorough internal inspection of the cylinder is required. If you don't want to unscrew the valve and look into the depths of your gas cylinders with electric lamps to make sure that no oil has got there, do not forget about the need for residual pressure.
All articles
On our website you can order the equipment you need in cities and regions: Moscow, St. Petersburg, Novosibirsk, Yekaterinburg, Nizhny Novgorod, Kazan, Chelyabinsk, Rostov-on-Don, Ufa, Krasnoyarsk, Krasnodar.
8(800)500-49-17 — Calls within Russia are free
gazzachas.ru
Methods for producing oxygen
Oxygen is mainly obtained in three ways:
- air separation by low-temperature rectification (deep cooling);
- decomposition of water by electrolysis (passing electric current);
- chemical method.
It is obtained from atmospheric air by deep cooling, as a by-product in the production of nitrogen.
O2 is also produced by passing an electric current through water (water electrolysis) and producing hydrogen along the way.
The chemical method of production is low-productive and, therefore, uneconomical; it has not found wide application and is used in laboratory practice.
Probably many people remember the chemical experiment when potassium permanganate (potassium permanganate KMnO4) is heated in a flask, and then the gas released during the heating process is collected in another flask?
2KMnO4 = K2MnO4 + MnO2 + O2 ↑
And the whole trick was when a smoldering splinter was placed in this flask and it flared up with a bright flame and the teacher explained that the gas released was O2, which supported combustion. And that the combustion process is nothing more than an oxidation process.
Characteristics
The containers are made of steel pipe with a strength limit of at least 65 kg/sq. mm, and the inner surface has no defects and is absolutely smooth. The wall thickness is no less than 6 mm and no more than 9 mm, on one side the product has a rounded shape, and on the other there is a sealed valve to which the gearbox is connected.
Filling oxygen cylinders is carried out only in a vertical position, for which a shoe is installed in the lower part . After filling, a safety cap is placed on the valve to protect the neck from mechanical damage during transportation.
Maximum pressure
Cylinders of different capacities can withstand a certain load when oxygen is pumped into them:
- 40 l - 150 kg/cm²;
- 50 l - 200 kg/cm².
The wall thickness depends on the manufacturer, but it is recommended not lower than the standard 6-8 mm.
Weight
How much a filled oxygen cylinder weighs is not so difficult to calculate, knowing the mass of all components:
- shoe - no more than 5.2 kg;
- rubber ring - 300 g;
- metal protective cap - 1.8 kg;
- the mass of the injected gas, depending on the pressure, is 8–12 kg.
After some quick calculations, we have the following result: a 40-liter filled cylinder weighs 67 kg, and a 50-liter cylinder weighs no more than 100 kg, which depends on the cap, which is also made of durable plastic.
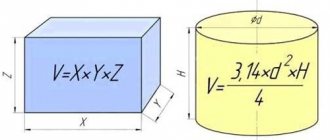
Volume
In order to find out how much oxygen is pumped into a cylinder per m3, there are special formulas, so a container designed for 40 liters can hold at a pressure of 150 kg/cm² - 150x40 = 6 thousand liters or 6 cubic meters. meters. For a 50 liter cylinder with a pressure of 200 kg/cm2 we get 10 m3.
Equipment
The main additional element of the oxygen tank is the valve. It is made from brass. A protective cap must be installed over the valve; it can be aluminum or plastic. Usually the cap comes as an integral part. But they are often lost, so the protective device can be made from any material with your own hands. Reliability and tightness are important here. The valve is screwed into the cylinder itself using a conical thread
The second most important element is the shoe. It is on him that the entire weight load falls. It is made from steel strip, which is shaped into a square cross-section. GOST does not define exactly how it should be fixed to the cylinder, so some manufacturers weld it, others press it in.
History of the discovery of oxygen
The discovery of oxygen is credited to Joseph Priestley. He had a laboratory equipped with instruments for collecting gases. He tested its physiological effects on himself and on mice. Priestley found that after inhaling the gas, a pleasant lightness was felt for some time. Mice in a hermetically sealed jar of air suffocate faster than in a jar of O2. Since Priestley was an adherent of the phlogiston theory, he never found out what was in his hands. He only described this gas, without even realizing what he was describing. But the laurels of the discovery of oxygen belong to Antoine Laurent de Lavoisier, who gave it its name.
Lavoisier staged his famous experiment, which lasted 12 days. He heated mercury in a retort. When boiling, its red oxide was formed. When the retort was cooled, it turned out that the air in it had decreased by almost 1/6 of its volume, and the remainder of the mercury weighed less than before heating. But when the mercury oxide was decomposed by strong calcination, everything returned: both the lack of mercury and the “disappeared” oxygen.
Subsequently, Lavoisier established that this gas is part of nitric, sulfuric, and phosphoric acids. He mistakenly believed that O2 was necessarily part of acids, and therefore called it “oxygenium,” which means “acid-producing.” Nowadays, acids devoid of “oxygenium” are well known (for example: hydrochloric, hydrogen sulfide, hydrocyanic, etc.).
Scope of use
Compressed oxygen is a popular gas, the scope of which depends on the type of raw material. The medical substance is used during the resuscitation of patients. The element has a beneficial effect on the heart and lungs, so therapeutic procedures are often prescribed for health problems. The component is taken to saturate cocktails during oxygen starvation.
Areas of use Source krsk.au.ru
Technical gas heats up quickly and maintains high temperatures for a long time. The resulting continuous jet burns through metal of any density, which allows you to cut or solder parts. The characteristic is useful both in construction and in domestic use. In metallurgy, the substance increases the efficiency of furnaces, thereby improving the quality of the finished product.
In the chemical industry they are used in the production of complex acids and explosives. In the pulp industry, paper is cleaned and bleached with oxygen; in the fish industry, ponds are enriched. In aviation, gas is involved in the oxidation of motor fuel.
Application in construction Source ugra.ru