Iron-carbon alloys
1.1. Iron
Iron belongs to the main chemical element in the group of ferrous metals.
Iron does not occur in its pure form in nature, but occurs in the form of oxides that form the so-called iron ore with varying iron content. The crystal lattice of iron is bcc, but it changes with increasing temperature. When heated to a temperature of 150 ... 250 ° C, pure iron, interacting with oxygen, becomes covered with an oxide film. Technically pure iron is ductile. With insignificant mass fractions of impurities (no more than 0.01%), ductility sharply decreases. Pure iron does not find practical use due to its low mechanical properties.
The metallurgical industry produces several types of technically pure iron for scientific research: pure, electrolytic, carbon, technical.
These grades of pure iron have different mechanical properties. For example, tensile strength σв = 180 ... 320 MPa (18 ... 32 kgf/mm2), relative elongation δ = 30 ... 50% depend on the type of iron. The ductility of iron depends on the test temperature.
When heated slightly, pure iron becomes brittle. Research has shown that iron has three zones of fragility:
- up to 270 °C - cold-brittle iron;
- 540 ... 720 °C - blue-brittle iron;
- 1,080 ... 1,265 °C - red-brittle iron.
The brittleness properties of iron manifest themselves depending on the type of atomic crystal lattice and its parameters. Chemical elements (impurities) increase or decrease the brittleness temperature of iron. For example, oxygen increases, and carbon and manganese decrease the brittleness temperature. In addition, practice shows that the strength and ductility of iron depend on the method of production of the samples under study. Cast samples have reduced strength and ductility compared to samples obtained by deformation (rolling, forging). Wrought iron is stronger than cast iron.
Iron in the solid state, depending on the temperature, can be in two modifications: α-iron and γ-iron. When heated and cooled, allotropic transformations occur, as a result of which the atomic lattices are rearranged. In addition, an important factor is the ability of iron to dissolve carbon, nitrogen and hydrogen, as well as various metals. In this case, substitutional solid solutions are formed with metals, and interstitial solutions with carbon, nitrogen and hydrogen.
Carbon, which dissolves in both α-iron and γ-iron, plays a special role in the formation of interstitial solid solutions. The highest solubility of carbon in γ-iron is 2.14% (on the Fe-Fe3C phase diagram, the critical temperature is 1,147 °C).
It is almost impossible to obtain pure iron. Currently, iron is obtained with a mass fraction of various impurities from 0.01%. For example, Armco iron - industrial iron produced in open-hearth furnaces and used for scientific research - has a mass fraction of impurities of 0.1 ... 0.2%.
Practice shows that carbon, when alloyed with iron, contributes to a sharp increase in mechanical properties - hardness, wear resistance, elasticity and strength increase. Plasticity and toughness decrease. In this regard, alloys of iron with carbon and other elements have found widespread use in practice.
1.2. Characteristics of iron-carbon alloys
An iron-carbon alloy is an alloy of iron that is saturated with carbon and other chemical elements during blast furnace smelting or another metallurgical process. Iron-carbon alloys are the main structural materials and represent a large group, both in terms of production volume and the variety of different grades.
Iron-carbon alloys, according to their physical, chemical and mechanical properties, are divided into two large groups: steels and cast irons.
Steel is an alloy of iron and carbon, in which the mass fraction of carbon is up to 2.14% (theoretically). In practice, the mass fraction of carbon is usually 1.3 ... 1.5%. Carbon steel is a deformable, malleable and strong structural material, the tensile strength of which reaches 1,150 MPa, hardness - 285 HB and elongation - 32%. The steel has good machinability, weldability and pressure treatment.
Cast iron is an alloy of iron and carbon, in which the mass fraction of carbon is 2.14 ... 6.67% (theoretically). In practice, cast irons with a carbon mass fraction of 2.5 ... 5.0% are used. The properties of cast iron depend on its structure, i.e., on the interaction of carbon with iron.
Depending on the structure, cast iron will have certain physical and mechanical properties. If it has a grain structure, cast iron will have high hardness and strength, as well as high machinability.
Most commercial pig iron is processed into steel.
1.3. Phases and structures of iron-carbon alloys.
An iron-carbon alloy, depending on physical conditions (temperature, pressure), can be in three phase states:
- liquid phase,
- semi-liquid phase,
- solid phase.
Each of these phases, despite the homogeneity of the chemical composition, has different physical and mechanical properties. When studying the properties of iron-carbon alloys, of scientific and practical interest are the various structures of the solid phase, which, when interacting with carbon, form iron (for example, the chemical compound cementite, solid solutions of austenite and ferrite, mechanical mixtures of pearlite and ledeburite).
Cementite is a chemical compound of carbon with iron (iron carbide) Fe3C. The structure of cementite is a complex orthorhombic atomic crystal lattice with a weak metallic bond. The temperature of the beginning of cementite melting is 1,147 °C, the end of melting is 1,600 °C. Cementite with various chemical elements found in iron-carbon alloys forms substitutional solid solutions. Under certain conditions (temperature and high pressure), cementite breaks down into free carbon (graphite).
Cementite is a ferromagnetic material, has no allotropic changes, and at a temperature of 217 ° C loses its magnetic properties. The amount of carbon in cementite is always constant and amounts to 6.67%. Cementite is the hardest (700 ... 800 HB) and brittle structural component of iron-carbon alloys; it cuts glass and cannot be processed by cutting. The plasticity of cementite is zero. When fractured, cementite has a fine-grained or needle-like structure, a white shiny color and is a typical structure of white pig iron.
Rice. 1. Microstructure of carbon steels: a - hypoeutectoid steel - ferrite + pearlite; b - eutectoid steel - pearlite; c - hypereutectoid steel - pearlite + secondary cementite
Ferrite is a solid solution of intercalated carbon in α-iron. This is low-carbon iron with a carbon mass fraction of up to 0.02%. Ferrite has a hardness of 60 ... 80 HB, tensile strength σв = 250 MPa (25 kgf/mm2), elongation at break δ = 50% and is the microstructure of low-carbon steels, which consists of large light grains.
Austenite is a solid solution of interstitial carbon in γ-iron.
The maximum carbon concentration in austenite is 2.14%, hardness is 170 ... 220 HB. Austenite is formed at a temperature of 727 °C and has an unstable structure; it disintegrates when cooled. The austenite structure has high mechanical (strength, ductility, toughness, hardness) and technological properties.
Perlite is a mechanical mixture of a solid solution of ferrite and the chemical compound cementite. This mixture is called eutectoid (easily disintegrating). Perlite has a fine-plate or granular structure.
The mass fraction of carbon in pearlite is 0.83%, hardness is 200 ... 240 HB, tensile strength σв = 1,100 ... 1,150 MPa (110 ... 115 kgf/mm2). Pearlite is an unstable structure; at a temperature of 727 °C it decomposes into austenite.
Ledeburite is a mechanical mixture of austenite and cementite. Ledeburite (eutectic) contains 4.3% carbon and melts at a constant temperature of 1,147 °C.
Steel with a carbon mass fraction of 0.83% is called eutectoid, less than 0.83% is hypoeutectoid, and more than 0.83% is hypereutectoid. The microstructures of eutectoid (pearlite), hypoeutectoid (ferrite + pearlite) and hypereutectoid (pearlite + secondary cementite) steels are shown in Fig. 1.
Cast iron with a carbon mass fraction of 4.3% is called eutectic, with a carbon mass fraction of less than 4.3% - hypoeutectic, with a carbon mass fraction of more than 4.3% - hypereutectic (Fig. 2).
Rice. 2. Microstructure of white cast iron: a - hypoeutectic cast iron - pearlite + ledeburite + cementite;
b - eutectic cast iron - ledeburite; c - hypereutectic cast iron - ledeburite + primary cementite
Structure and properties
The main phase that initiates the nucleation of ledeburite is cementite. A flat austenite dendrite grows on a cementite plate nucleated in the eutectic fluid. This is followed by a relatively rapid pairwise growth of mutually germinated crystals of both phases. Each of the phases within one ledeburite colony is continuous, that is, it belongs to one crystal.
Depending on the temperature, the phase composition of ledeburite can be different. Thus, in the temperature range from 1147 °C to 727 °C, ledeburite consists of austenite and cementite, and at temperatures below 727 °C - of ferrite and cementite.
Ledeburite has high hardness and brittleness.
The influence of chemical elements on the properties of iron-carbon alloys
An iron-carbon alloy, in addition to iron and carbon (permanent components), contains useful, harmful and permanent (technological) impurities, which have various effects on the properties of structural materials.
Carbon in an iron-carbon alloy can be in the form of iron carbide Fe3C (cementite) or in the form of graphite.
Carbon is the main alloyed component, which is specially introduced to increase strength, hardness and improve the technological and operational properties of structural materials. The influence of carbon on the properties of structural materials depends on the state or interaction of it with iron, i.e., on the formation of a particular structure of the iron-carbon alloy.
If cementite is formed during the interaction of carbon with iron, then the alloy will have high hardness, brittleness and will practically not be machinable by cutting.
If carbon, interacting with iron, forms structures of mechanical mixtures (pearlite or ferrite), then the alloy will have high mechanical and technological properties.
Silicon and manganese in iron-carbon alloys are useful impurities. Silicon enters the alloy partially from the ore, and the bulk comes during the smelting process during deoxidation of the alloy.
Deoxidation is the process of removing harmful iron oxide (FeO) inclusions by introducing deoxidizers (manganese, silicon and various ferroalloys). Deoxidizers combine with iron oxide to form slags that must be removed. A small part of the deoxidizers remains in the iron-carbon alloy. As a rule, most steels and cast irons are subject to deoxidation.
Silicon completely dissolves in the main structure of the alloy to form a solid solution, increases the yield strength, and reduces brittleness. High silicon content (1 ... 2%) gives steel elasticity. In addition, silicon promotes the conversion of carbon from the cementite structure into free carbon in the form of graphite, thereby reducing the hardness and brittleness of the alloys. Manganese enters the alloy during the processing of manganese ores, as well as during the deoxidation process.
Manganese forms a solid solution with iron and also promotes the formation of a chemical compound, so it increases hardness, wear resistance, and strength. High manganese content promotes the formation of cementite, which leads to increased hardness and brittleness of the alloy. In addition, manganese neutralizes the harmful effects of sulfur.
Sulfur and phosphorus in iron-carbon alloys are harmful impurities.
Phosphorus enters the alloy from the ore. The original cast iron, as a rule, has a high mass fraction of phosphorus. Phosphorus has limited dissolution in iron, and its excess content leads to the formation of iron phosphide, a very fragile compound. Phosphorus, dissolving in iron, sharply reduces its density and leads to brittleness in a cold state. This property is called the cold brittleness of alloys. Phosphorus also leads to increased hardness and decreased strength. Manganese, interacting with phosphorus in the process of deoxidation, removes it with the formation of slags.
In some cases, phosphorus can be useful, as it improves machinability, fluidity, and, in the presence of a small amount of copper, increases corrosion resistance.
Sulfur enters the alloy from ores, as well as from fuel during its combustion. Without dissolving in iron, sulfur forms a fusible and very brittle mechanical mixture (eutectic) with it and makes the alloy brittle at red-hot temperatures (this property is called red-brittleness), therefore iron-carbon alloys with a high sulfur content are not subjected to hot pressure treatment.
At a high carbon content in an alloy, the presence of sulfur increases its hardness and brittleness, worsens casting properties, reduces fluidity, increases metal shrinkage when cooling castings and the tendency to form microcracks. The harmful effects of sulfur are neutralized by manganese. When a small mass fraction of manganese is introduced into the alloy, a compound with sulfur is formed - manganese sulfide (MnS) instead of low-melting iron sulfide (FeS). Manganese sulfide is partially removed along with the slag.
Iron-carbon alloys can contain various gases in very low mass fractions: nitrogen, hydrogen and oxygen. These chemical elements are hidden impurities. Due to the complexity of their chemical analysis, the mass fraction of these elements is not determined and is not standardized in technical specifications.
In addition, various metals (tin, zinc, antimony, lead, nickel, copper, chromium, etc.) - random impurities - are found in small quantities. These groups of metals come from both ores and scrap steel processed during the metallurgical process.
All of the listed random impurities are an inevitable consequence of the technological process, i.e. they are not added on purpose. In this regard, the resulting steels with an insignificant mass fraction of nickel, copper, chromium and other metals are not considered as alloy steels.
In addition to natural, permanent, hidden and random chemical elements, special chemical elements are introduced into an iron-carbon alloy (especially steel) in order to change the microstructure of the alloy, physical-chemical and other properties.
Chemical elements specially introduced into an iron-carbon alloy are called alloying elements, and alloys obtained on their basis are called alloy alloys (steels and cast irons).
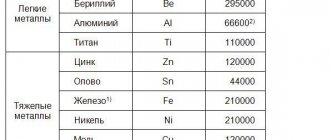
Aluminum, vanadium, tungsten, molybdenum, copper, cobalt, silicon, nickel, tantalum, titanium, chromium, etc. are introduced as alloying elements.
State standards strictly regulate the mass fraction of useful and harmful impurities in iron-carbon alloys.
In steels, as a rule, the content of these elements, %, is limited to the following upper limits:
Manganese………………………………………………………………………………… 0.8
Silicon ………………………………………………………………………………….. 0.5
Phosphorus………………………………………………………………………………… 0.05
Sulfur…………………………………………………………………………………… 0.05
In cast iron, according to state standards, a higher mass fraction, %, of useful and harmful impurities is allowed (depending on the groups and brands):
Manganese ………………………………………………………………………………… 0.3 … 1.5
Silicon ………………………………………………………………………………… 0.3 … 5.0
Phosphorus………………………………………………………………………. 0.20… 0.65
Sulfur………………………………………………………………………………. 0.08…0.12
Structure and properties
The main phase that initiates the nucleation of ledeburite is cementite. A flat austenite dendrite grows on a cementite plate nucleated in the eutectic fluid. This is followed by a relatively rapid pairwise growth of mutually germinated crystals of both phases. Each of the phases within one ledeburite colony is continuous, that is, it belongs to one crystal.
Depending on the temperature, the phase composition of ledeburite can be different. Thus, in the temperature range from 1147 °C to 727 °C, ledeburite consists of austenite and cementite, and at temperatures below 727 °C - of ferrite and cementite.
Ledeburite has high hardness and brittleness.
Iron - Carbon phase diagram.
Reading an Iron-Carbon Diagram
We can see the composition of an alloy with a given initial carbon content at a given temperature by moving along a vertical line corresponding to the carbon content in the alloy.
Consider, for example, the AEC region. It is adjacent to areas of austenite AESG and the liquid phase. The alloys in it consist of a liquid phase and the resulting solid austenite. How to determine the carbon concentration in different phases for a given alloy? Let us consider, as an example, an alloy with an initial carbon concentration of 2.5% at a temperature of 1250°C.
Let’s draw a horizontal straight line from this point on the graph “2.5% C – 1250°C”. The intersection of this straight line with the AE line bordering the austenite region will show the carbon concentration in austenite at a given temperature (~1.5%).
The intersection of the same horizontal line with line AC, bordering the liquid phase region, will show the concentration of carbon in the liquid phase at a given temperature (~3.5%).
This is how we can determine the carbon concentration in the phases of any alloy at a given temperature:
- in the liquid phase and austenite in the AEC region;
- in the liquid phase in the CDF region (carbon concentration in cementite, of course, is constant - 6.67%);
- in austenite in the SEFK region;
- in ferrite in the QPKL region;
- in ferrite and austenite in the GPS region.
As we can see, at a carbon concentration above 2.14%, the saturation of the cooled melt with carbon always tends to 4.3% (along the AC and DC lines) as it approaches a temperature of 1147°C (ECF level). Next, the liquid transforms into ledeburite (eutectic). Naturally, with the same average carbon content.
As the temperature approaches 727°C (PSK level), the carbon concentration in austenite (“free” and/or included in ledeburite) tends to 0.8% (according to the GS and ES lines). Next, the transformation of austenite into pearlite (eutectoid) occurs. Perlite, of course, has an average carbon content of 0.8%.
Structure and properties
The main phase that initiates the nucleation of ledeburite is cementite. A flat austenite dendrite grows on a cementite plate nucleated in the eutectic fluid. This is followed by a relatively rapid pairwise growth of mutually germinated crystals of both phases. Each of the phases within one ledeburite colony is continuous, that is, it belongs to one crystal.
Depending on the temperature, the phase composition of ledeburite can be different. Thus, in the temperature range from 1147 °C to 727 °C, ledeburite consists of austenite and cementite, and at temperatures below 727 °C - of ferrite and cementite.
Ledeburite has high hardness and brittleness.
Structure and properties
The main phase that initiates the nucleation of ledeburite is cementite. A flat austenite dendrite grows on a cementite plate nucleated in the eutectic fluid. This is followed by a relatively rapid pairwise growth of mutually germinated crystals of both phases. Each of the phases within one ledeburite colony is continuous, that is, it belongs to one crystal.
Depending on the temperature, the phase composition of ledeburite can be different. Thus, in the temperature range from 1147 °C to 727 °C, ledeburite consists of austenite and cementite, and at temperatures below 727 °C - of ferrite and cementite.
Ledeburite has high hardness and brittleness.
Structure and properties
The main phase that initiates the nucleation of ledeburite is cementite. A flat austenite dendrite grows on a cementite plate nucleated in the eutectic fluid. This is followed by a relatively rapid pairwise growth of mutually germinated crystals of both phases. Each of the phases within one ledeburite colony is continuous, that is, it belongs to one crystal.
Depending on the temperature, the phase composition of ledeburite can be different. Thus, in the temperature range from 1147 °C to 727 °C, ledeburite consists of austenite and cementite, and at temperatures below 727 °C - of ferrite and cementite.
Ledeburite has high hardness and brittleness.