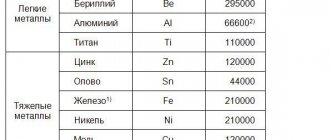
What is an alloy
Let's look at this issue in more detail. So, an alloy is a combination of several metals or one and various non-metallic additives. Such connections are used everywhere. An alloy is a macroscopic homogeneous system obtained by melting. They have been known since ancient times, when humanity, using primitive technologies, learned to produce cast iron, bronze, and a little later - steel.
The production and use of these materials is due to the fact that it is possible to obtain an alloy with specified technological properties, while many characteristics (strength, hardness, corrosion resistance, and others) are higher than those of its individual components.
Intermetallic alloys
The composition of intermetallic compounds is usually expressed by formulas that are completely incompatible with the valences that metals exhibit in compounds with metalloids. For example, sodium forms the following series of compounds with tin and lead:
NaSn6NaSn4, NaSn3, NaSn2, NaSn, Na4Sn2, Na2Sn, Na3Sn, Na4Sn, NaPb, Na2Pb, Na4Pb
Many intermetallic compounds are very strong and do not decompose at temperatures well above their melting points.
Intermetallic compounds have the ability to dissolve in liquid ammonia, forming conductive solutions. During the electrolysis of such solutions, one of the metals, less electropositive, is released at the anode, the other at the cathode. For example, during the electrolysis of a Na4Pb9 solution, lead is released at the anode and sodium at the cathode.
In solutions, intermetallic compounds can enter into exchange reactions with various salts. For example:
2Ca(NO3)2+ K4Pb =Ca2Pb + 4KNO3
Metals can be displaced from intermetallic compounds by other metals in exactly the same way as from ordinary salts.
The formation of intermetallic compounds has not yet been theoretically explained. It has only been established that metals with very similar chemical properties usually do not form compounds with each other.
Recently, in the study of alloys, X-ray analysis methods have been widely used, which make it possible to establish the internal structure of the crystals that form the alloy and determine their crystal lattices.
The properties of alloys differ in many ways from the properties of the metals being fused, and are by no means their arithmetic averages, due to the formation of various chemical compounds or solid solutions during fusion.
The melting point of alloys is very often lower than the melting point of the most fusible component of the alloy. An example of an alloy with a very low melting point is an alloy consisting of lead (4 parts), tin (2 parts), bismuth (6 parts) and cadmium (1 part). It melts at about 75°, that is, already when lowered into hot water, while the melting point of the most fusible of the four named metals - tin - is 231.9°. On the contrary, the hardness of alloys is usually greater than the hardness of their constituent parts, especially if during alloying chemical compounds of metals are formed, which, as a rule, are harder than the metals being alloyed, but also more brittle. Alloys containing solid solutions have very high hardness.
203 204 205
You are reading an article on the topic Alloys
Main types
How are alloys classified? This is done according to the type of metal that is the basis of the connection, namely:
- Black. The base is iron. Ferrous alloys include all types of steels and cast irons.
- Colored. The base is one of the non-ferrous metals. The most common non-ferrous alloys are based on copper and aluminum.
- Rare metal alloys. Based on vanadium, niobium, tantalum, tungsten. They are used mainly in electrical engineering.
- Alloys of radioactive metals.
Other elements - metals and non-metals - are added to the main component of the alloy, which improve its technological properties. These additives are called alloying additives. Alloys also contain harmful impurities - if their permissible value is exceeded, many of the material’s characteristics are reduced. So now you know what an alloy is.
Alloys are also classified into double, triple and others - according to the number of components. According to the homogeneity of the structure - homogeneous and heterogeneous. According to their distinctive properties - low-melting and refractory, high-strength, heat-resistant, anti-friction, corrosion-resistant and materials with special properties.
Alloys. Types, characteristics of alloys
Due to their low mechanical properties, pure metals as structural materials in mechanical engineering are of limited use compared to alloys.
An alloy is a structural material obtained by fusing several chemical elements (metals and non-metals) and possessing properties inherent in the main element being alloyed.
A metal alloy can be obtained not only by fusion of chemical elements, but also by such methods as sintering, electrolysis, diffusion, plasma spraying, sublimation, etc. A structural material not obtained by fusion is called a pseudo-alloy. If an alloy contains 50% metal or more, then it is called a metal alloy.
A metal alloy has higher mechanical and technological properties compared to a non-metal alloy. The chemical elements that form an alloy are called components.
In terms of their composition, alloys can be two-component (metal + metal, metal + non-metal), three-component or more. The internal structure of alloys is determined by the form of bonding between the components.
Two-component alloys, when heated (cooled), due to the peculiarities of their interaction with each other, behave inadequately and, therefore, have different physical structures and properties.
Industrial alloys that are widely used include:
- cast iron and steel are alloys of iron and carbon;
- brass - an alloy of copper and zinc;
- bronze - an alloy of copper and tin, etc.
Alloys have an atomic-crystalline structure, have allotropy (polymorphism) and, in comparison with pure metals, higher mechanical and technological properties.
The form of the metallic bond of the chemical elements being fused affects the formation of the alloy structure and their atomic-crystalline lattice.
Phases. Alloys, like pure metals, are characterized by an atomic-crystalline structure. An alloy in the solid state may have a different connection of atomic-crystal lattices. The liquid or solid state of chemical components that form an alloy at a certain temperature and pressure is called a system.
The homogeneous part of the system, separated from other parts by a conventional boundary (line), is called a phase.
The liquid phase is characterized by the fact that the atomic crystal lattices of the alloyed components disintegrate and the components dissolve in each other or do not dissolve and are present in the alloy independently. This pattern is inherent in many alloys.
The solid phase is a homogeneous part of the alloy with a certain atomic-crystalline structure and mass fraction of alloyed components. The atomic lattices of the fused components interact in a strictly defined order. The atomic lattices of the chemical elements that form the alloy in the solid state form small crystals - structures.
Depending on the internal structure of the alloys and the metallic or chemical bond between the alloyed elements, alloys are divided into two groups:
- homogeneous alloys;
- heterogeneous alloys.
Homogeneous alloys have common atomic crystal lattices, which include atoms of the alloyed components.
Inhomogeneous alloys have independent crystal lattices of alloyed components.
Based on the nature of the interaction of the fused components in the solid phase, mechanical mixtures, solid solutions and chemical compounds are distinguished.
A mechanical mixture of fused components A and B (Fig. 1, a) is formed when the atomic crystal lattices are preserved and do not enter into a chemical reaction to form any new compound. The connection between atomic lattices is carried out due to metallic bonds. The mechanical mixture of the alloy will be of a heterogeneous type, that is, the alloyed components A and B in the alloy will be independent and alternate with each other depending on their ratio.
Rice. 1. Schematic representation of the alloy structure: a - mechanical mixture; b - solid solution (I - substitution solution; II - interstitial solution); c - chemical compound; A, B - fused components
The properties of the mechanical mixture depend on the properties of the fused components A and B. As a rule, these microstructures have relatively high hardness, strength, impact strength, and are well processed by cutting.
Solid solutions , depending on the interaction of atoms, are divided into interstitial solid solutions and substitutional solid solutions (Fig. 1, b).
In Fig. 1, b, I shows the atomic crystal lattice of a substitutional solid solution. The atomic-crystal lattice of the main component A in the form of a body-centered cube (nine atoms) is preserved, but three atoms of this component are replaced by atoms of the fused component B.
In Fig. Figure 1, b, II shows the atomic crystal lattice of the interstitial solid solution. With this type of alloy formation, the atomic crystal lattice of the main component A is preserved. The atomic-crystal lattice of the alloyed component B is destroyed, and its individual atoms are introduced into the space of the atomic-crystal lattice of the main component A. Thus, in the atomic-crystal lattice of the interstitial solid solution there are nine atoms, as in the main component A, plus two or three atoms of the component B.
Solid solutions in their properties are closest to the properties of the main component. They have low hardness, high density, impact strength, strength, and are easily deformed in cold and hot conditions. The microstructure of most structural and tool steels is interstitial and substitutional solid solutions.
Chemical compounds of the alloy are formed when the atomic crystal lattices of the alloyed components A and B disintegrate. Individual atoms of these components form new atomic lattices, which in their type, shape and number of atoms differ from the atomic crystal lattices of the fused components.
Chemical compounds in the alloy are formed at a strictly defined mass ratio of the alloyed components A and B. For example, a chemical compound of carbon with iron is formed at a mass fraction of carbon equal to 6.67%.
The properties of chemical compounds also differ sharply from the properties of the fused components. Chemical compounds are usually very hard, brittle, refractory, and have a fine-grained or needle-like microstructure. In Fig. 1, c shows an atomic-crystalline cell of a chemical compound of carbon with iron. This is a complex rhombic spatial atomic-crystal lattice, consisting of iron atoms and carbon atoms (components A and B).
In practice, most often a mixture of several compounds (microstructures) is observed in an alloy, for example, a mechanical mixture of a chemical compound and a solid solution or a mechanical mixture of two solid solutions.
State diagrams of two-component alloys. Any change in the chemical composition of the alloy entails a change in physical parameters: temperature, pressure and structure. The change in these parameters at phase boundaries occurs abruptly or slowly.
In the practice of metallurgy, graphs - state diagrams of alloys - are used to determine temperatures, pressure, structure and interaction of alloyed components. To do this, the alloy is heated (cooled) in a closed crucible using a thermocouple, the behavior of this alloy is observed using the device, and corresponding graphs are drawn from the observations.
Phase diagrams display only conditions when the alloy has constant parameters - equilibrium, therefore in the scientific literature, phase diagrams are also called equilibrium diagrams. Due to the fact that the alloyed components (metals and non-metals) have allotropy, when heated (cooled) allotropic changes occur in the alloys. Allotropic changes can be observed in laboratory studies using the thermal method, and sometimes visually (the color of the alloy becomes brighter or, conversely, dims, or remains constant for a long time).
Any change in a metal during heating (cooling) is characterized by a certain temperature, which is called the critical temperature. Critical temperatures on a straight line are reflected by corresponding points, which are called critical points. If we consider any metal or alloy in one dimension (heating temperature), then the graphical characteristic will be displayed in the form of a vertical line on which critical temperatures (points) are indicated. If the state of a metal or alloy is considered in two dimensions (heating (cooling) temperature and heating (cooling) time), then the graph will be depicted in two coordinates (ordinate axis and abscissa axis).
For example, consider the state of pure iron when heated and cooled. In Fig. Figure 2 shows the critical temperatures of pure iron when heating (cooling). Iron has the following critical points (temperatures): 768; 910; 1,392 and 1,539 °C. At a temperature of 910 °C, Fe-α (α-iron) transforms into Fe-β (β-iron). At a temperature of 1,392 °C, Fe-β transforms into Fe-γ (γ-iron). At a temperature of 1539 °C, Fe-γ begins to slowly melt with the absorption of energy (temperature).
At all critical temperatures, the diagrams show recrystallization delays (horizontal sections). When iron cools, the recrystallization process occurs in the reverse order.
For two-component alloys, a phase diagram is a graphical representation of the state of the alloys in two dimensions: heating (cooling) temperature and chemical composition of the alloy (concentration).
Rice. 2. Iron heating and cooling curves: t - temperature; τ — time
The heating (cooling) temperature is plotted along the ordinate axis, and the mass fraction of the fused components (concentration) is plotted along the abscissa axis.
For example, consider the phase diagram of a two-component lead-antimony alloy (Fig. 3). On the x-axis we take 100% lead (Pb) on the left, and 100% antimony (Sb) on the right. Lead and antimony in the liquid state dissolve indefinitely in each other, in the solid state they form a mechanical mixture of fused components.
When an alloy is heated (cooled) from the solid state to the melting temperature (and when cooled from the liquid state to the solidification temperature), the formation of mechanical mixtures (eutectic) and melting at different temperatures occurs in the alloy.
Let's take pure lead. At normal temperatures and up to a temperature of 245 ° C, no changes in the internal structure occur in lead, and the lead will have the structure Pb-α (α-lead). At a temperature of 245 °C, Pb-α is transformed into Pb-β (β-lead). This structure remains up to a temperature of 327 °C.
At a temperature of 327 °C, lead begins to melt. When melting due to the absorption of energy (temperature), the temperature of the lead remains constant - 327 ° C. When cooling lead, the process occurs in the reverse order.
Rice. 3. Cooling curves and structures (a, b, c, e, f), state diagram (d) of lead-antimony alloys: 1 - liquidus temperature; 2—solidus temperature; ABC - liquidus line; DBE—solidus line; F - liquid; Eut. - eutectic
When antimony is heated to a temperature of 245 °C, no changes occur in the metal. The structure of antimony will be Sb-α (α-antimony). At a temperature of 245 °C, Sb-α transforms into Sb-β. At a temperature of 631°C, antimony begins to melt. Due to the fact that during melting there is a large absorption of heat, the melting temperature of antimony will be 8 ... 10 ° C lower. When cooling, the process occurs in the reverse order. Next, we consider the behavior of typical lead and antimony alloys: 95% Pb + 5% Sb; 87% Pb + 13% Sb; 60% Pb + 40% Sb. To draw a state diagram of a two-component lead-antimony alloy, we construct heating (cooling) curves.
When heating (cooling) 100% Pb (Fig. 3, a) at a temperature of 327 °C, there will be a horizontal section on the graph. When heating (cooling) the alloy 95% Pb + 5% Sb (Fig. 3, b) at a temperature of 245 °C, there will be a horizontal section on the graph. Further, when heated (cooled) at a temperature of 300 ° C, there will be an inflection in the curve; at this temperature, the alloy will begin to melt (when heated) or crystallize (when cooled). When heating (cooling) the alloy 87% Pb + 13% Sb (Fig. 3, c) at a temperature of 245 °C there will also be a horizontal section. At this temperature the alloy begins to melt and finishes melting at a temperature of 245 °C.
When heating (cooling) the alloy 60% Pb + 40% Sb (Fig. 3, e) to a temperature of 245 °C, no changes occur in the structure of the alloy. At a temperature of 245 °C, lead begins to melt - there will be a horizontal section on the graph. With further heating (cooling) at a temperature of 350 °C, the alloy melts (when heated) or begins to crystallize (when cooled).
When 100% of antimony (Fig. 3, e) is heated (cooled) to a temperature of 631 °C, the alloy will have a solid phase, and at a temperature of 631 °C there will be a horizontal section on the graph, antimony begins to melt. Due to the absorption of energy, the melting of antimony occurs at a temperature slightly below 631 ° C.
To visualize the characteristics of the lead-antimony alloy, we construct the following graph. On the ordinate axis we plot the heating (cooling) temperatures from the normal temperature. On this axis we will plot the critical points for 100% lead. On the abscissa axis we plot the mass fraction of lead and antimony in the alloy. On the right we draw the temperature axis for 100% antimony content. Next, on the ordinate axis we project the critical points obtained as a result of heating the previously considered alloys.
As we can see from the graphs, the first phase change of the alloys occurs at a temperature of 245 °C. We draw a horizontal straight line DE corresponding to this temperature. On the lead temperature axis we project a point corresponding to a temperature of 327 °C - the melting point of pure lead. Let us denote the resulting point by the letter A.
On the antimony temperature axis we project a point corresponding to 631 °C - the melting point of antimony. Let us denote the resulting point by the letter C. On the x-axis, from the point corresponding to 87% Pb and 13% Sb, restore the perpendicular (dotted line) to the horizontal straight line DE (melting temperature of this alloy). Point A (critical temperature 327 °C) on the ordinate axis is connected to the critical point lying on the horizontal line corresponding to the melting point of this alloy (87% Pb + 13% Sb). Let us denote the resulting point by the letter B.
On the abscissa axis, from the point corresponding to 95% Pb and 5% Sb, we restore the perpendicular to the intersection with the segment AB. At this point we have a critical temperature of 300 °C - the melting (solidification) temperature of the alloy 95% Pb + 5% Sb.
On the abscissa axis, from the point corresponding to 60% Pb and 40% Sb, we restore the perpendicular to the intersection with the segment BC, we obtain a point that corresponds to the critical temperature of 350 ° C - melting (solidification) of the alloy 60% Pb + 40% Sb.
Thus, we have obtained a phase diagram of the two-component lead-antimony alloy. All Pb-Sb alloys, regardless of the mass fraction of components, up to a temperature of 245 °C have a solid phase - a mechanical mixture. The alloy along the DBE line begins to slowly melt when heated and solidifies when cooled. This line is called the solidus line (from the Latin solidus - solid).
Along the ABC line, alloys melt when heated, and when cooled they begin to slowly crystallize. This line is called the liquidus line (from the Latin liquidus - liquid). Between the DBE lines and the ABC line, the alloys are in a semi-liquid state. An alloy with 87% Pb and 13% Sb has the lowest melting (solidification) temperature. This alloy, like pure metals, melts at one temperature. Such alloys are called eutectic alloys.
Eutectic is a finely dispersed mechanical mixture of two components formed at a melting temperature (crystallization) significantly lower than the melting temperature of the fused components during the solidification process. Alloys to the left of the eutectic are called hypoeutectic, and those to the right are called hypereutectic.
Let us consider the phase states of the lead-antimony alloy. Above the ABC line the alloy is in a liquid state (liquid phase), between the AB and DB lines it is in a semi-liquid state (Pb + liquid). Below the DB line, the alloy consists of a mechanical mixture of lead and eutectic. Between the lines BC and BE the alloy will have a semi-liquid phase and antimony crystals. Below line BE, the alloy will consist of a mechanical mixture (eutectic and antimony).
The phase diagram of the Pb - Sb alloy refers to the type of diagrams in which the alloyed components dissolve indefinitely in the liquid state and do not dissolve in the solid state, forming mechanical mixtures (eutectic).
By analyzing the state diagram of alloys, you can study the following characteristics: melting (crystallization) temperature, types of alloy structures, ability to form segregation, heat treatment and pressure treatment modes. When studying the phase diagrams of two-component alloys, attention should be paid to the transformation of the alloy components in the crystalline (solid) state.
In this regard, the following features of allotropic changes in alloys are distinguished (typical phase diagrams):
- phase diagrams of the first kind - for alloys, the components of which are completely dissolved in the liquid state, limitedly dissolved in the solid state and form mechanical mixtures (Pb-Sb, Sn-Zn, etc.);
- phase diagrams of the second kind - for alloys, the components of which completely dissolve in the liquid and solid states with the formation of solid solutions (Ag-Au, Cu-Ni; Fe-V, etc.);
- phase diagrams of the third kind - for alloys, the components of which are unlimitedly soluble in the liquid state, practically insoluble in the solid state and form mechanical mixtures (eutectic) with a polymorphic transformation (primary and secondary) of the structural-phase composition;
- phase diagrams of the fourth kind - for alloys, the components of which in the liquid state dissolve in each other, and in the solid state form stable or unstable chemical compounds.
728
Mechanical properties
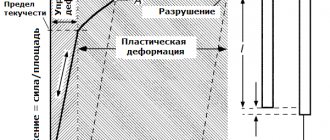
The mechanical properties of alloys determine the performance of the material when exposed to external forces. In order to determine the characteristics of the connection, the sample is subjected to various tests (stretched, scratched, loaded, a metal ball or diamond cone is pressed into it, studied under a microscope) to determine strength, elasticity, and ductility.
Alloy as a technological process
This process is an integral part of logging operations and is a type of transportation of timber (logs) by water. Depending on the waterway along which logging is carried out, as well as on the time of year, rafting can be:
- prayer;
- raft;
- purse.
The first type of rafting was used earlier during spring floods in hard-to-reach sections of rivers and was a simple fusion of logs not connected to each other. This type has been banned in the Russian Federation since 1995, because rafted logs move chaotically along the river, which can lead to congestion of river beds. The second method of rafting is carried out during periods when rivers are unnavigable or poorly navigable. With this option, the logs are tied into a raft (or a bundle) and they must be accompanied by small vessels (boats or small motor boats). The third type of rafting is used only at times when rivers are completely navigable. Its essence lies in the fact that the logs are cut to the same size and formed into separate batches, fenced with floating walls.
Each such fenced lot is called a “purse” and its rafting is always controlled by a medium-sized river fleet.
Chemical
What are the chemical properties of the alloy? These are characteristics that determine how a material reacts to the effects of various active, including aggressive, agents. The chemical effect of the environment can be seen visually: iron is “eaten up” by rust, a green coating of oxides appears on bronze, steel dissolves in sulfuric acid.
In metallurgy and heavy engineering, many methods are used to combat the aggressive influence of the external environment: new, more resistant materials based on copper, titanium and nickel are being developed, alloys are coated with protective layers - varnishes, paints, oxide films, and their structure is improved. As a result of negative environmental factors, industry annually suffers damage amounting to millions of tons of steel and cast iron.
Story
One of the most famous places where bronze items were found was located in the Kuban River area. In this place, archaeologist Nikolai Veselovsky in 1897 excavated the so-called Maykop culture, which existed in the second half of the 4th millennium BC.
Bronze artifacts found in the Maikop burial mounds were made mainly from an alloy of copper and arsenic, so it is believed that historically these alloys, called arsenic bronzes, .
It was in no way inferior in its properties to alloys of copper with tin or lead, and even surpassed them in a number of characteristics . It was widely used in various areas of human activity of those times, from the manufacture of critical parts to jewelry.
Bronze composition
Bronze is an alloy of copper with metals such as tin, aluminum, lead, beryllium, and non-metals arsenic, silicon and phosphorus. In addition, such alloys can be additionally alloyed with phosphorus, zinc, manganese, iron and nickel.
The composition of bronze depends on the grade of the alloy and is indicated in its designation. For example, the composition of an alloy labeled BrAMts7−1 includes 7% aluminum, 1% manganese and 92% copper .
Thus, the main component of this metal is copper (from 35% to 90% and above). The second component can be either arsenic, or tin or beryllium, lead, aluminum, silicon and other components. To impart special properties, additional components can be added - zinc, iron, nickel, manganese, phosphorus and others.
Technological
Manufacturability – what is it? In industry, an alloy is not needed on its own; it is used to make a part. Consequently, the material will be heated, cut, deformed, subjected to heat treatment and other manipulations. Manufacturability is the ability of an alloy to be subjected to various methods of hot and cold processing, for example, melting, easily spreading and filling a casting mold, deforming in hot or cold form (forging, hot and cold stamping), welding, and processing with metal-cutting tools.
Technological properties can be divided into:
- Foundry. They are characterized by fluidity - the ability to fill the mold for casting, shrinkage (the percentage of volume loss after cooling, hardening) and segregation - a complex process in which a heterogeneous structure of the material is formed in different parts of the casting.
- Ductility. This is the ability of an alloy to deform under impact load and take the desired shape without loss of integrity. Some metals have good malleability only when hot, others - both cold and hot. For example, steel is forged while hot. Aluminum alloys and brass take the desired shape well at room temperature. Bronze is difficult to deform by impact, and cast iron is not ductile and is destroyed under the influence of a hammer (with the exception of malleable cast iron).
- Weldability. Low-carbon steel has good weldability; this characteristic is much worse for high-alloy steels and cast irons.
Excerpt characterizing the Alloy
- I have the honor to report, Mr. Colonel, there are only eight charges, would you order to continue firing? - he asked. - Buckshot! - Without answering, the senior officer shouted, looking through the rampart. Suddenly something happened; The officer gasped and, curling up, sat down on the ground, like a shot bird in flight. Everything became strange, unclear and cloudy in Pierre’s eyes. One after another, the cannonballs whistled and hit the parapet, the soldiers, and the cannons. Pierre, who had not heard these sounds before, now only heard these sounds alone. To the side of the battery, on the right, the soldiers were running, shouting “Hurray,” not forward, but backward, as it seemed to Pierre. The cannonball hit the very edge of the shaft in front of which Pierre stood, sprinkled earth, and a black ball flashed in his eyes, and at the same instant it smacked into something. The militia who had entered the battery ran back. - All with buckshot! - the officer shouted. The non-commissioned officer ran up to the senior officer and in a frightened whisper (as a butler reports to his owner at dinner that there is no more wine required) said that there were no more charges. - Robbers, what are they doing! - the officer shouted, turning to Pierre. The senior officer's face was red and sweaty, his frowning eyes sparkling. – Run to the reserves, bring the boxes! - he shouted, angrily looking around Pierre and turning to his soldier. “I’ll go,” said Pierre. The officer, without answering him, walked in the other direction with long steps. – Don’t shoot... Wait! - he shouted. The soldier, who was ordered to go for the charges, collided with Pierre. “Eh, master, there’s no place for you here,” he said and ran downstairs. Pierre ran after the soldier, going around the place where the young officer was sitting. One, another, a third cannonball flew over him, hitting in front, from the sides, from behind. Pierre ran downstairs. "Where am I going?" - he suddenly remembered, already running up to the green boxes. He stopped, undecided whether to go back or forward. Suddenly a terrible shock threw him back to the ground. At the same instant, the brilliance of a large fire illuminated him, and at the same instant a deafening thunder, crackling and whistling sound rang in his ears. Pierre, having woken up, was sitting on his backside, leaning his hands on the ground; the box he was near was not there; only green burnt boards and rags were lying on the scorched grass, and the horse, shaking its shaft with fragments, galloped away from him, and the other, like Pierre himself, lay on the ground and squealed shrilly, protractedly. Pierre, unconscious from fear, jumped up and ran back to the battery, as the only refuge from all the horrors that surrounded him. While Pierre was entering the trench, he noticed that no shots were heard at the battery, but some people were doing something there. Pierre did not have time to understand what kind of people they were. He saw the senior colonel lying with his back to him on the rampart, as if examining something below, and he saw one soldier he noticed, who, breaking forward from the people holding his hand, shouted: “Brothers!” – and saw something else strange. But he had not yet had time to realize that the colonel had been killed, that the one shouting “brothers!” There was a prisoner who, in front of his eyes, was bayoneted in the back by another soldier. As soon as he ran into the trench, a thin, yellow, sweaty-faced man in a blue uniform, with a sword in his hand, ran at him, shouting something. Pierre, instinctively defending himself from the push, since they, without seeing, ran away from each other, put out his hands and grabbed this man (it was a French officer) with one hand by the shoulder, with the other by the proud. The officer, releasing his sword, grabbed Pierre by the collar. For several seconds, they both looked with frightened eyes at faces alien to each other, and both were at a loss about what they had done and what they should do. “Am I taken prisoner or is he taken prisoner by me? - thought each of them. But, obviously, the French officer was more inclined to think that he had been taken prisoner, because Pierre’s strong hand, driven by involuntary fear, squeezed his throat tighter and tighter. The Frenchman wanted to say something, when suddenly a cannonball whistled low and terribly above their heads, and it seemed to Pierre that the French officer’s head had been torn off: he bent it so quickly. Pierre also bowed his head and let go of his hands. Without thinking any more about who took whom prisoner, the Frenchman ran back to the battery, and Pierre went downhill, stumbling over the dead and wounded, who seemed to him to be catching his legs. But before he had time to go down, dense crowds of fleeing Russian soldiers appeared towards him, who, falling, stumbling and screaming, ran joyfully and violently towards the battery. (This was the attack that Ermolov attributed to himself, saying that only his courage and happiness could have accomplished this feat, and the attack in which he allegedly threw the St. George crosses that were in his pocket onto the mound.) The French who occupied battery, let's run. Our troops, shouting “Hurray,” drove the French so far beyond the battery that it was difficult to stop them.
Alloys, their classification and properties
There are several ways to classify alloys:
- by manufacturing method (cast and powder alloys);
- by the method of obtaining the product (casting, wrought and powder alloys);
- by composition (homogeneous and heterogeneous alloys);
- according to the nature of the metal - base (ferrous - Fe base, non-ferrous - base, non-ferrous metals and alloys of rare metals - radioactive elements base);
- by the number of components (double, triple, etc.);
- by characteristic properties (refractory, low-melting, high-strength, heat-resistant, hard, anti-friction, corrosion-resistant, etc.);
- by purpose (structural, instrumental and special).
What to pay attention to during rafting
1. Always wear a life jacket when on the river and make sure it fits snugly to your body. Even if you are a good swimmer, you cannot neglect this safety measure.
2. Remember that helmets are primarily intended to protect you from the paddles of enthusiastic rafting companions and very rarely as protection from rocks on the river. However, since there is such a danger, do not remove your helmet until the end of the rafting and make sure that it is securely fastened to your head.
3. Do not tie/wrap your wrists, arms, legs or neck with rope! It could be dangerous if you fall into the water.
4. Do not place your feet on the safety rope outside the raft while paddling. So you could get hurt if you hit a boulder.
5. If the raft is about to hit a boulder, do not try to stop the two-ton momentum of the inflatable with your light paddle, foot or hands, as this may cause injury.
6. Stop water fights and other fun activities in front of rapids and make sure that personal protective equipment - a life jacket and helmet - fit snugly to the body.
7. If you fall into the water, do not panic and relax: the life jacket will immediately bring you to the surface. There is about a 90% chance that you will surface right next to the raft. Grab it. Remember that the inflatable is your biggest life jacket on the river.
If you find yourself away from the raft, lie on your back with your feet pointing downstream. Do not try to stand up suddenly, as you may catch your foot on a rock or other underwater obstacle. Listen carefully to your guide, even if his instructions contradict your instincts - swim there and as he says.
8. Have fun! If you raft together with an experienced guide and strictly follow his instructions, not forgetting about safety measures, then rafting will bring a lot of bright impressions, and you will probably want to repeat it!
Problems for independent solution.
Simple problems with two components of the mixture.
1-1.
A mixture of copper and aluminum weighing 20 g was treated with a 96% solution of nitric acid, and 8.96 liters of gas (n.e.) were released. Determine the mass fraction of aluminum in the mixture.
1-2.
A mixture of copper and zinc weighing 10 g was treated with a concentrated alkali solution. In this case, 2.24 liters of gas (n.y.) were released. Calculate the mass fraction of zinc in the initial mixture.
1-3.
A mixture of magnesium and magnesium oxide weighing 6.4 g was treated with a sufficient amount of dilute sulfuric acid. In this case, 2.24 liters of gas (n.s.) were released. Find the mass fraction of magnesium in the mixture.
1-4.
A mixture of zinc and zinc oxide weighing 3.08 g was dissolved in dilute sulfuric acid. We obtained zinc sulfate weighing 6.44 g. Calculate the mass fraction of zinc in the original mixture.
1-5.
When a mixture of iron and zinc powders weighing 9.3 g was exposed to an excess solution of copper (II) chloride, 9.6 g of copper was formed. Determine the composition of the initial mixture.
1-6.
What mass of a 20% solution of hydrochloric acid will be required to completely dissolve 20 g of a mixture of zinc and zinc oxide, if hydrogen is released with a volume of 4.48 l (n.s.)?
1-7.
When 3.04 g of a mixture of iron and copper is dissolved in dilute nitric acid, nitrogen oxide (II) is released with a volume of 0.896 l (n.s.). Determine the composition of the initial mixture.
1-8.
When 1.11 g of a mixture of iron and aluminum filings was dissolved in a 16% solution of hydrochloric acid (ρ = 1.09 g/ml), 0.672 liters of hydrogen (n.s.) were released. Find the mass fractions of metals in the mixture and determine the volume of hydrochloric acid consumed.
The tasks are more complex.
2-1.
A mixture of calcium and aluminum weighing 18.8 g was calcined without air with an excess of graphite powder. The reaction product was treated with dilute hydrochloric acid, and 11.2 liters of gas (n.o.) were released. Determine the mass fractions of metals in the mixture.
2-2.
To dissolve 1.26 g of magnesium-aluminium alloy, 35 ml of a 19.6% sulfuric acid solution (ρ = 1.1 g/ml) was used. The excess acid reacted with 28.6 ml of potassium bicarbonate solution with a concentration of 1.4 mol/l. Determine the mass fractions of metals in the alloy and the volume of gas (no.) released during the dissolution of the alloy.
2-3.
When 27.2 g of a mixture of iron and iron (II) oxide was dissolved in sulfuric acid and the solution was evaporated to dryness, 111.2 g of iron sulfate—iron (II) sulfate heptahydrate—was formed. Determine the quantitative composition of the initial mixture.
2-4.
When iron weighing 28 g reacted with chlorine, a mixture of iron (II) and (III) chlorides weighing 77.7 g was formed. Calculate the mass of iron (III) chloride in the resulting mixture.
2-5.
What was the mass fraction of potassium in its mixture with lithium, if as a result of treating this mixture with excess chlorine, a mixture was formed in which the mass fraction of potassium chloride was 80%?
2-6.
After treating a mixture of potassium and magnesium with a total mass of 10.2 g with excess bromine, the mass of the resulting mixture of solids turned out to be equal to 42.2 g. This mixture was treated with an excess of sodium hydroxide solution, after which the precipitate was separated and calcined to constant weight. Calculate the mass of the resulting residue.
2-7.
A mixture of lithium and sodium with a total mass of 7.6 g was oxidized with excess oxygen, a total of 3.92 l (n.s.) was consumed. The resulting mixture was dissolved in 80 g of 24.5% sulfuric acid solution. Calculate the mass fractions of substances in the resulting solution.
2-8.
An aluminum-silver alloy was treated with an excess of a concentrated solution of nitric acid, and the residue was dissolved in acetic acid. The volumes of gases released in both reactions, measured under the same conditions, turned out to be equal. Calculate the mass fractions of metals in the alloy.
Three metals and complex problems.
3-1.
When 8.2 g of a mixture of copper, iron and aluminum was treated with an excess of concentrated nitric acid, 2.24 liters of gas were released. The same volume of gas is released when the same mixture of the same mass is treated with an excess of dilute sulfuric acid (DS). Determine the composition of the initial mixture in mass percent.
3-2.
14.7 g of a mixture of iron, copper and aluminum, interacting with an excess of dilute sulfuric acid, releases 5.6 liters of hydrogen (n.s.). Determine the composition of the mixture in mass percent if chlorination of the same sample of the mixture requires 8.96 liters of chlorine (n.s.).
3-3.
Iron, zinc and aluminum filings are mixed in a molar ratio of 2:4:3 (in the order listed). 4.53 g of this mixture was treated with excess chlorine. The resulting mixture of chlorides was dissolved in 200 ml of water. Determine the concentrations of substances in the resulting solution.
3-4.
An alloy of copper, iron and zinc weighing 6 g (the masses of all components are equal) was placed in an 18.25% solution of hydrochloric acid weighing 160 g. Calculate the mass fractions of substances in the resulting solution.
3-5.
13.8 g of a mixture consisting of silicon, aluminum and iron were treated with excess sodium hydroxide when heated, and 11.2 liters of gas (n.s.) were released. When such a mass of mixture is exposed to excess hydrochloric acid, 8.96 liters of gas (n.s.) are released. Determine the masses of substances in the original mixture.
3-6.
When a mixture of zinc, copper and iron was treated with an excess of concentrated alkali solution, gas was released, and the mass of the undissolved residue turned out to be 2 times less than the mass of the original mixture. This residue was treated with an excess of hydrochloric acid, the volume of gas released turned out to be equal to the volume of gas released in the first case (the volumes were measured under the same conditions). Calculate the mass fractions of metals in the initial mixture.
3-7.
There is a mixture of calcium, calcium oxide and calcium carbide with a molar ratio of components of 3:2:5 (in the order listed). What is the minimum volume of water that can enter into a chemical reaction with such a mixture weighing 55.2 g?
3-8.
A mixture of chromium, zinc and silver with a total mass of 7.1 g was treated with dilute hydrochloric acid, the mass of the undissolved residue turned out to be 3.2 g. After separating the precipitate, the solution was treated with bromine in an alkaline medium, and at the end of the reaction it was treated with excess barium nitrate. The mass of the formed precipitate turned out to be equal to 12.65 g. Calculate the mass fractions of metals in the initial mixture.
Sports alloy
Any sport requires training, often special, as it involves risk to life. This is especially true for sports rafting aimed at passing obstacles. This requires both endurance and muscle strength. There are several types of this tourism:
- river rafting;
- extreme;
- free style on a stormy river.
In rowing rafting, athletes compete. The judges take into account the speed of the distance and its accuracy. If a hanging gate is hit, a penalty will be assessed. Freestyle, or freestyle as it is called, involves performing tricks in rough water. It also takes into account the number and complexity of figures that athletes manage to make in a certain time.