For pure substances (elements), the liquidus and solidus temperatures coincide. For solutions, which include steel and cast iron, there is a so-called crystallization temperature range in which solid and liquid phases simultaneously coexist.
Melting temperatures of steel
Under certain conditions, solids melt, that is, they turn into a liquid state. Each substance does this at a certain temperature.
- Melting is the process of transition of a substance from a solid to a liquid state.
- Melting point is the temperature at which a crystalline solid melts into a liquid state. Denoted by t.
Physicists use a specific table of melting and crystallization, which is given below:
| Substance | t,°C | Substance | t,°C | Substance | t,°C |
| Aluminum | 660 | Copper | 1087 | Alcohol | — 115 |
| Voden | — 256 | Naphthalene | 80 | Cast iron | 1200 |
| Tungsten | 3387 | Tin | 232 | Steel | 1400 |
| Iron | 1535 | Paraffin | 55 | Titanium | 1660 |
| Gold | 1065 | Mercury | — 39 | Zinc | 420 |
Based on the table, we can safely say that the melting point of steel is 1400 °C.
Refractory metals
The temperature of refractory metals is above 1600°C. These are tungsten, titanium, platinum, chromium and others. They are used as light sources, machine parts, lubricants, and in the nuclear industry. They are used to make wires, high-voltage wires, and are used to melt other metals with a lower melting point. Platinum begins to transition from solid to liquid at a temperature of 1769 degrees, and tungsten at a temperature of 3420°C.
Mercury is the only metal that is in a liquid state under normal conditions, namely, normal atmospheric pressure and average ambient temperature. The melting point of mercury is minus 39°C. This metal and its vapors are poisonous, so it is used only in closed containers or in laboratories. A common use of mercury is as a thermometer to measure body temperature.
Each metal and alloy has its own unique set of physical and chemical properties, not least of which is the melting point. The process itself means the transition of a body from one state of aggregation to another, in this case, from a solid crystalline state to a liquid one. To melt a metal, it is necessary to apply heat to it until the melting temperature is reached. With it, it can still remain in a solid state, but with further exposure and increased heat, the metal begins to melt. If the temperature is lowered, that is, some of the heat is removed, the element will harden.
The highest melting point among metals belongs to tungsten : it is 3422C o, the lowest is mercury: the element melts at -39C o. As a rule, it is not possible to determine the exact value for alloys: it can vary significantly depending on the percentage of components. They are usually written as a number interval.
Stainless steel
Stainless steel is one of the many iron alloys found in steel. It contains Chromium from 15 to 30%, which makes it rust-resistant, creating a protective layer of oxide on the surface, and carbon. The most popular brands of this type are foreign. These are the 300th and 400th series. They are distinguished by their strength, resistance to adverse conditions and ductility. The 200 series is of lower quality, but cheaper. This is a beneficial factor for the manufacturer. Its composition was first noticed in 1913 by Harry Brearley, who conducted many different experiments on steel.
At the moment, stainless steel is divided into three groups:
- Heat-resistant - at high temperatures it has high mechanical strength and stability. The parts that are made from it are used in the pharmaceutical, rocketry, and textile industries.
- Rust-resistant - has great resistance to rusting processes. It is used in household and medical devices, as well as in mechanical engineering for the manufacture of parts.
- Heat-resistant - resistant to corrosion at high temperatures, suitable for use in chemical plants.
The melting point of stainless steel varies depending on its grade and the number of alloys from approximately 1300 °C to 1400 °C.
Alloy types
Depending on the intensity of heating required for the transition of a metal from one state to another, alloys are divided into several types.
Low fusible. They can be processed even without special equipment. The melting point of steel in degrees Celsius is 600. Low-melting metals include lead, tin and zinc.
Mercury deserves special attention because it can turn into a liquid state at -39°C.
Medium melting. The melting point of steel is in the range of 600°C-1600°C. This category includes aluminum, copper, tin, some types of stainless steel and various alloys with a small chromium content. Medium-melting compounds are most widespread in industry and in everyday life.
Refractory. Compounds included in this category are capable of transitioning from solid to liquid when heated above 1600°C. These are highly alloyed metals, which include tungsten, titanium and chromium. Thanks to these additives, the metal acquires increased strength, resistance to corrosion and chemical influences. In particular, stainless steel is a refractory alloy.
Alkali metals melt at the lowest temperatures. Accordingly, for non-alkali metals to transition into the liquid state, the temperature range increases significantly.
Boiling degree
When heating a material, it is important not to reach its boiling point, at which point it goes from a liquid state to a gaseous state. Therefore, the boiling point is an equally important technological indicator.
The boiling point is typically twice the degree at which materials melt and is determined at normal atmospheric pressure. As the pressure increases, the heating intensity also increases. As the pressure decreases, the readings decrease.
Features of Carbon Steel
Carbon compounds are the main type of products produced at metallurgical plants. In addition to iron, they also contain carbon. Its concentration should not exceed 2.14%. They contain a small amount of impurities and alloying components in the form of manganese, silicon and magnesium. Such additives can improve their physical and chemical properties.
Depending on the carbon concentration, carbon compounds are divided into the following types:
- low-carbon (carbon content does not exceed 0.29%);
- medium carbon (up to 0.6%);
- high carbon (more than 0.6%).
Carbon compounds are used in various industrial sectors. Depending on the scope of application, alloying components are added to them to achieve specific properties, including heat resistance, corrosion resistance, etc. According to these criteria, they are divided into the following categories:
- structural;
- instrumental.
Manganese is added to tool tools, which significantly improves the quality of the metal. The melting point of carbon steel is 1535°C.
Features of Alloy Steel
Additional components are introduced into the composition of alloyed compounds. In certain quantities they give them the required properties. Depending on the concentration of such elements, they are divided into the following types:
- low-alloyed (with a concentration of 2.5%);
- medium alloyed (up to 10%);
- highly alloyed (over 10%).
By adding additional components, it is possible to increase strength, corrosion resistance and improve other characteristics. The alloying components are chromium, copper, nickel, nitrogen, vanadium, etc. The melting point of alloy steel ranges from 1400°C to 1480°C.
Features of stainless steel
Stainless steel is an alloy that is resistant to dry and wet corrosion and is immune to aggressive substances. To give it the necessary properties, various alloying components are added to the metal in the form of chromium, nickel, magnesium, titanium, etc. The melting point of stainless steel in Celsius is 1350-1500 degrees.
Below is a table showing the melting point of heat-resistant stainless steel of the most popular brands.
| Brand | t°С |
| 12Х18Г9 | 1410 |
| Х20Н35 | 1410 |
| 12Х18Н9Т | 1425 |
| Х25С3Н | 1480 |
| 15Х25Т | 1500 |
Features of tool steel
This material is intended exclusively for the manufacture of tools. It differs from the structural one by an increased carbon content of more than 0.7%. Such compounds are mainly used in mechanical engineering for processing ferrous metal and non-ferrous metal. The melting point of stainless steel intended for the manufacture of tools is 1500°C.
Cast iron and steel
Cast iron is an alloy of carbon and iron, it contains impurities of manganese, silicon, sulfur and phosphorus. Withstands low voltages and loads. One of its many advantages is its low cost for consumers. There are four types of cast iron:
- White - has high strength and poor ability to be processed with a knife. Types of alloy according to the increase in the amount of carbon in the composition: hypoeutectic, eutectic, hypereutectic. It was called white due to the fact that it has a white color in the fault. White cast iron also has a special structure of the metal mass and great wear resistance. Useful in making mechanical parts that will operate in a non-lubricated environment. It is used to make the following types of cast iron.
- Gray cast iron - contains carbon, silicon, manganese, phosphorus and some sulfur. It can be easily obtained and has poor mechanical properties. Used for the manufacture of parts that are not exposed to shock loads. There is a gray color in the fracture; the darker it is, the softer the material. The properties of gray cast iron depend on the temperature of the environment in which it is located and the amount of various impurities.
- Malleable cast iron is obtained from white cast iron as a result of simmering (prolonged heating and holding). The substance contains: carbon, silicon, manganese, phosphorus, and a small amount of sulfur. It is more durable and ductile, easier to process.
- Ductile iron is the strongest of all types of cast iron. Contains carbon, manganese, sulfur, phosphorus, silicon. Has high impact strength. This important metal is used to make pistons, crankshafts and pipes.
The melting points of steel and cast iron are different, as stated in the table above. Steel has higher strength and resistance to high temperatures than cast iron, temperatures differ by as much as 200 degrees. For cast iron, this number ranges from approximately 1100 to 1200 degrees, depending on the impurities it contains.
Density, melting and boiling points of simple substances: tables for elements
The table shows the basic physical properties of simple substances: density at a temperature of 20 ° C (if the density is measured at another temperature, the latter is indicated in parentheses), melting point and boiling point of substances in degrees Celsius.
The density and melting and boiling points of the following simple substances are indicated: nitrogen N2, actinium Ac, aluminum Al, americium Am, argon Ar, astatine At, barium Ba, beryllium Be, boron B, bromine Br, vanadium V, bismuth Bi, hydrogen h3, tungsten W, gadolinium Gd, gallium Ga, hafnium Hf, helium He, germanium Ge, holmium Ho, dysprosium Dy, europium Eu, iron Fe, gold Au, indium In, iodine (iodine) J, iridium Ir, ytterbium Yb, yttrium Y , cadmium Cd, potassium K, calcium Ca, oxygen O2, ozone O3, cobalt Co, silicon Si, krypton Kr, xenon Xe, curium Cm, lanthanum La, lithium Li, lutetium Lu, magnesium Mg, manganese Mn, copper Cu, molybdenum Mo, arsenic As, sodium Na, neodymium Nd, neon Ne, neptunium Np, nickel Ni, niobium Nb, tin Sn, osmium Os, palladium Pd, platinum Pt, plutonium Pu, polonium Po, praseodymium Pr, promethium Pm, protactinium Pa, radium Ra, radon Rn, rhenium Re, rhodium Rh, mercury Hg, rubidium Rb, ruthenium Ru, samarium Sm, lead Pb, selenium Se, sulfur S, silver Ag, scandium Sc, strontium Sr, antimony Sb, thallium Tl, tantalum Ta , tellurium Te, terbium Tb, technetium Tc, titanium Ti, thorium Th, thulium Tu, carbon C (diamond, graphite), uranium U, phosphorus P (white, red), francium Fr, fluorine F, chlorine Cl, chromium Cr, cesium Cs, cerium Ce, zinc Zn, zirconium Zr, erbium Er.
Read also: Homemade spot welding from a microwave, wire cross-section
It should be noted that the density of substances in the table is expressed in units of kg/m3. In the table you can highlight substances (chemical elements) with minimum and maximum densities. Gases have the lowest density of chemical elements - for example, the density of hydrogen is only 0.08987 kg/m3 - this is the lightest gas on the planet. Of the heavy elements, tungsten, uranium, neptunium, osmium and other metals are distinguished by high density.
The numbers in brackets mean that the substance decomposes at a given temperature. Abbreviations: g. - gas, l. - liquid, tv. - solid, sublime — sublimates, rhombus. - rhombic structure.
According to the table, it is possible to identify substances with minimum and maximum melting and boiling points. The chemical element helium has the lowest melting point - its melting point is minus 272.2 °C. Helium also has the lowest boiling point.
The chemical element carbon in the form of graphite has the highest melting point among simple substances. It begins to melt at a temperature of 3600°C. Another modification of carbon, diamond, is also a refractory substance with a melting point of 3500°C.
The element cadmium has the highest boiling point; it boils at a temperature not lower than 7670°C, although it begins to melt at only 321°C.
Atomic mass and density of simple substances
The table shows the atomic mass and density of the following chemical elements: nitrogen, actinium, aluminum, americium, argon, astatine, barium, beryllium, berkelium, boron, bromine, vanadium, bismuth, hydrogen, tungsten, gadolinium, gallium, hafnium, helium, germanium , holmium, dysprosium, europium, iron, gold, indium, iodine, iridium, ytterbium, yttrium, cadmium, potassium, californium, calcium, oxygen, cobalt, silicon, krypton, xenon, curium, lanthanum, lithium, lutetium, magnesium, manganese , copper, mendelevium, molybdenum, arsenic, sodium, neodymium, neon, neptunium, nickel, niobium, tin, osmium, palladium, platinum, plutonium, polonium, praseodymium, promethium, protactinium, radium, radon, rhenium, rhodium, mercury, rubidium , ruthenium, samarium, lead, selenium, sulfur, silver, scandium, strontium, antimony, thallium, tantalum, tellurium, terbium, technetium, titanium, thorium, thulium, carbon (graphite, diamond), uranium, fermium, phosphorus, francium, fluorine, chlorine, chromium, cesium, cerium, zinc, zirconium, einsteinium, erbium.
The densities indicated correspond to the densities of substances at a temperature of 20°C and atmospheric pressure, except in cases where a different temperature is indicated in parentheses.
The density of elements is given in the dimension of ton per cubic meter. For example, the density of liquid nitrogen at a temperature of -195.8°C is 0.808 t/m3 or 808 kg/m3; The density of chlorine in the gaseous state is 3.214 kg/m3, and that of liquid chlorine is 1557 kg/m3. The density values of substances are given for their natural molecular and aggregate states at the indicated temperature.
Sources:1. Pisarenko V.V. Handbook of laboratory chemist. Ref. manual for professional technical textbook establishments. M., “Higher School”, 1970. - 192 pages with illustration 2. Physical quantities. Directory. A.P. Babichev, N.A. Babushkina, A.M. Bratkovsky and others; Ed. I.S. Grigorieva, E.Z. Meilikhova. - M.: Energoatomizdat, 1991. - 1232 p.
PHYSICAL PROPERTIES OF METALS MELTING POINT
The particles of any body - atoms or molecules - are in constant random motion. In solids, this movement is practically limited to the vibration of atoms around a certain equilibrium position. The higher the body temperature, the more animated the movement. At a certain temperature, a solid melts and turns into a liquid.
Amorphous bodies - wax, resin, amber, glass - when heated, gradually soften and then become liquid. The transition of wax from solid to liquid occurs smoothly, and we cannot say exactly what the melting point of wax is.
Crystalline substances are a different matter. When heated, the ions fixed at the nodes of the crystal lattice vibrate more and more energetically, but as long as the lattice is maintained, the crystal remains solid. Only when the vibrations of the ions increase so much that the lattice is destroyed do the first traces of liquid appear. That is why all crystalline substances, including metals, have a completely definite melting point.
Among the metals there are those for the melting of which special high-temperature electric furnaces are built; There are those that melt from the warmth of your hand, and there are those that melt at temperatures below zero.
The most fusible metals are mercury and cesium, and the most refractory are rhenium and tungsten. Below we provide a table of melting points of various metals:
Melting point in degrees Celsius
Melting point in degrees Celsius
The transfer of heat from one body to another is the transfer of energy of random motion from one molecules to another.
Water, glass, air, wood, brick transfer heat slowly, their thermal conductivity is low. Metals conduct heat very quickly. How can we explain this?
We already know that in the spatial lattice of metal crystals there are positively charged metal atoms - ions. They are more or less firmly held in place. Free electrons move randomly around the ions. They can be imagined as “electron gas” flowing around the crystal lattice. Free electrons easily move inside the lattice and serve as good carriers of heat energy from heated metal layers to cold ones.
The high thermal conductivity of a metal is always easy to detect. In cold weather, touch the wall of a wooden house and an iron fence with your hand: iron is always much colder to the touch than wood, since iron quickly removes heat from the hand, and wood is hundreds of times slower. Silver and gold conduct heat better than all other metals, followed by copper, aluminum, tungsten, magnesium, zinc and others. The worst metal conductors of heat are lead and mercury.
Read also: Jack for car repair
Thermal conductivity is measured by the amount of heat that passes through a metal rod with a cross-section of
1 square centimeter in 1 minute. If the thermal conductivity of silver is conventionally taken as 100, then the thermal conductivity of copper will be 90, aluminum 27, iron 15, lead 12, mercury 2, and the thermal conductivity of wood is only 0.05.
The greater the thermal conductivity of the metal, the faster and more evenly it heats up.
Due to their high thermal conductivity, metals are widely used in applications where rapid heating or cooling is required. Steam boilers, devices in which various chemical processes take place at high temperatures, central heating radiators, car radiators - all this is made of metals. Devices that must give or absorb a lot of heat are most often made of good heat conductors - copper, aluminum.
These sheet products reliably eliminate slipping on the surface of the material. Various corrugations are applied to the smooth side of the sheet in the form of a diamond, duet, lentil, quintet or any other pattern. But the fluting quintet and...
You can buy low-carbon steel AISI 310s online at a competitive price and with prompt delivery exclusively through the online service of manufacturers with a reputation as a responsible partner. Only in this case can you count...
Made from steel 12x18n10t, the stainless steel circle and mirror sheet are plastic materials with an impact-resistant structure that are resistant to intergranular corrosion.
Characteristics table
Metals and alloys are an indispensable basis for forging
, foundry production, jewelry production and many other areas of production.
Whatever the craftsman does ( gold jewelry
, cast iron fences, steel knives or
copper bracelets)
, in order to work correctly, he needs to know the temperatures at which this or that element melts.
To find out this parameter, you need to refer to the table. In the table you can also find the boiling degree.
Among the most commonly used elements in everyday life, the melting point indicators are as follows:
- aluminum – 660 °C;
- copper melting point – 1083 °C;
- gold melting point – 1063 °C;
- silver – 960 °C;
- tin – 232 °C. Tin is often used for soldering, since the temperature of a working soldering iron is exactly 250–400 degrees;
- lead – 327 °C;
- melting point of iron – 1539 °C;
- the melting point of steel (an alloy of iron and carbon) is from 1300 °C to 1500 °C. It varies depending on the saturation of the steel with components;
- melting point of cast iron (also an alloy of iron and carbon) – from 1100 °C to 1300 °C;
- mercury – -38.9 °C.
As is clear from this part of the table, the most fusible metal is mercury, which at positive temperatures is already in a liquid state.
Read also: What is an inverter motor in a washing machine?
The boiling point of all these elements is almost twice, and sometimes even higher than the melting point. For example, for gold it is 2660 °C, for aluminum
- 2519 °C
, for iron - 2900 °C, for copper - 2580 °C, for mercury - 356.73 °C.
For alloys such as steel, cast iron and other metals, the calculation is approximately the same and depends on the ratio of components in the alloy.
The maximum boiling point of metals is rhenium – 5596
°C
. The highest boiling point is for the most refractory materials.
There are tables that also indicate the density of metals
.
The lightest metal is lithium, the heaviest is osmium. Osmium has a higher density than uranium
and plutonium when viewed at room temperature. Light metals include: magnesium, aluminum, titanium. Heavy metals include most common metals: iron, copper, zinc, tin and many others. The last group is very heavy metals, these include: tungsten, gold, lead and others.
Another indicator found in the tables is the thermal conductivity of metals
. Neptunium is the worst conductor of heat, and the best metal in terms of thermal conductivity is silver. Gold, steel, iron, cast iron and other elements are in the middle between these two extremes. Clear characteristics for each can be found in the required table.
Melting point, along with density, refers to the physical characteristics of metals
.
The melting point of a metal
is the temperature at which a metal changes from its normal solid state (except mercury) to a liquid state when heated.
During melting, the volume of the metal practically does not change, so normal atmospheric pressure does not affect
.
The melting point of metals ranges from -39 degrees Celsius to +3410 degrees
. For most metals, the melting point is high, however, some metals can be melted at home by heating on a regular burner (tin, lead).
Classification of metals by melting point
- Low-melting metals
, the melting point of which ranges
up to 600
degrees Celsius, for example
zinc, tin, bismuth
. - Medium-melting metals
that melt at temperatures
from 600 to 1600
degrees Celsius: such as
aluminum, copper, tin, iron
. - Refractory metals
, the melting point of which reaches
more than 1600
degrees Celsius -
tungsten, titanium, chromium
, etc. - - the only metal that is in a liquid state under normal conditions (normal atmospheric pressure, average ambient temperature). The melting point of mercury is about -39 degrees
Celsius.
Table of melting temperatures of metals and alloys
650
1000
| Metal | |
| Aluminum | 660,4 |
| Tungsten | 3420 |
| Duralumin | |
| Iron | 1539 |
| Gold | 1063 |
| Iridium | 2447 |
| Potassium | 63,6 |
| Silicon | 1415 |
| Brass | |
| Low melting alloy | 60,5 |
| Magnesium | 650 |
| Copper | 1084,5 |
| Sodium | 97,8 |
| Nickel | 1455 |
| Tin | 231,9 |
| Platinum | 1769,3 |
| Mercury | –38,9 |
| Lead | 327,4 |
| Silver | 961,9 |
| Steel | 1300-1500 |
| Zinc | 419,5 |
| Cast iron | 1100-1300 |
When melting metal for the manufacture of metal castings, the choice of equipment, material for molding the metal, etc. depends on the melting temperature. It should also be remembered that when alloying the metal with other elements, the melting temperature most often decreases
.
Do not confuse the concepts of “metal melting point” and “metal boiling point” - for many metals these characteristics are significantly different: for example, silver melts at a temperature of 961 degrees Celsius, and boils only when the temperature reaches 2180 degrees.
The melting point of a metal is the minimum temperature at which it changes from solid to liquid. When melting, its volume practically does not change. Metals are classified by melting point depending on the degree of heating.
Melting point of metals
Melting of a metal is a certain thermodynamic process in which the crystal lattice of the metal is destroyed and it passes from the solid phase state to the liquid.
The melting point of metals is an indicator of the temperature of the heated metal, upon reaching which the process of phase transition (melting) begins. The process itself is the reverse of crystallization and is inextricably linked with it. To melt metal? it must be heated using an external heat source to the melting point, and then continue to supply heat to overcome the phase transition energy. The fact is that the very value of the melting point of metals indicates the temperature at which the material will be in phase equilibrium, at the boundary between a liquid and a solid. At this temperature, pure metal can exist simultaneously in both solid and liquid states. To carry out the melting process, it is necessary to overheat the metal slightly above the equilibrium temperature in order to provide a positive thermodynamic potential. Give a kind of impetus to the process.
The melting point of metals is constant only for pure substances. The presence of impurities will shift the equilibrium potential in one direction or another. This happens because a metal with impurities forms a different crystal lattice, and the forces of interaction between atoms in them will differ from those present in pure materials. Depending on the melting point, metals are divided into low-melting ones (up to 600 ° C, such as gallium , mercury), medium-melting (600-1600°C, copper, aluminum) and refractory (>1600°C, tungsten, molybdenum).
In the modern world, pure metals are rarely used due to the fact that they have a limited range of physical properties. Industry has long and extensively used various combinations of metals - alloys, the varieties and characteristics of which are much greater. The melting point of the metals that make up various alloys will also differ from the melting point of their alloy. Different concentrations of substances determine the order of their melting or crystallization. But there are equilibrium concentrations at which the metals that make up the alloy solidify or melt simultaneously, that is, they behave like a homogeneous material. Such alloys are called eutectic.
Knowing the melting point is very important when working with metal; this value is necessary both in production, for calculating the parameters of alloys, and during the operation of metal products, when the phase transition temperature of the material from which the product is made determines the restrictions on its use. For convenience, these data are summarized in a single table. The metal melting table is a summary result of physical studies of the characteristics of various metals. There are also similar tables for alloys. The melting point of metals also significantly depends on pressure, so these tables are relevant for a specific pressure value (usually these are normal conditions when the pressure is 101.325 kPa). The higher the pressure, the higher the melting point, and vice versa.
Metals have a number of original properties that are unique to these materials. There is a melting point for metals at which the crystal lattice is destroyed. The substance retains its volume, but it is no longer possible to talk about the constancy of its shape.
Individual metals are found extremely rarely in their pure form. In practice, alloys are used. They have certain differences from pure substances. When complex compounds are formed, the crystal lattices combine with each other. Therefore, the properties of alloys may differ markedly from those of their constituent elements. The melting point no longer remains constant; it depends on the concentration of the ingredients included in the alloy.
Characteristics table
Metals and alloys are an indispensable basis for forging , foundry, jewelry and many other areas of production. Whatever the craftsman does ( gold jewelry , cast iron fences, steel knives or copper bracelets) , in order to work correctly, he needs to know the temperatures at which this or that element melts.
To find out this parameter, you need to refer to the table. In the table you can also find the boiling degree.
Among the most commonly used elements in everyday life, the melting point indicators are as follows:
- aluminum - 660 °C;
- copper melting point - 1083 °C;
- melting point of gold - 1063 °C;
- silver - 960 °C;
- tin - 232 °C. Tin is often used for soldering, since the temperature of a working soldering iron is exactly 250–400 degrees;
- lead - 327 °C;
- melting point of iron - 1539 °C;
- the melting point of steel (an alloy of iron and carbon) is from 1300 °C to 1500 °C. It varies depending on the saturation of the steel with components;
- melting point of cast iron (also an alloy of iron and carbon) - from 1100 °C to 1300 °C;
- mercury - -38.9 °C.
As is clear from this part of the table, the most fusible metal is mercury, which at positive temperatures is already in a liquid state.
The boiling point of all these elements is almost twice, and sometimes even higher than the melting point. For example, for gold it is 2660 °C, for aluminum - 2519 °C , for iron - 2900 °C, for copper - 2580 °C, for mercury - 356.73 °C.
For alloys such as steel, cast iron and other metals, the calculation is approximately the same and depends on the ratio of components in the alloy.
Read also: Do-it-yourself machine for paving slabs drawings
The maximum boiling point of metals is rhenium - 5596 ° C. The highest boiling point is for the most refractory materials.
There are tables that also indicate the density of metals . The lightest metal is lithium, the heaviest is osmium. Osmium has a higher density than uranium and plutonium when viewed at room temperature. Light metals include: magnesium, aluminum, titanium. Heavy metals include most common metals: iron, copper, zinc, tin and many others. The last group is very heavy metals, these include: tungsten, gold, lead and others.
Another indicator found in the tables is the thermal conductivity of metals . Neptunium is the worst conductor of heat, and the best metal in terms of thermal conductivity is silver. Gold, steel, iron, cast iron and other elements are in the middle between these two extremes. Clear characteristics for each can be found in the required table.
Each metal or alloy has unique properties, including its melting point. In this case, the object passes from one state to another, in a particular case, it becomes liquid from solid. To melt it, you need to apply heat to it and heat it until the desired temperature is reached. At the moment when the desired temperature point of a given alloy is reached, it may still remain in a solid state. As exposure continues, it begins to melt.
Mercury has the lowest melting point - it melts even at -39 °C, tungsten has the highest - 3422 °C. For alloys (steel and others) it is extremely difficult to determine the exact figure. It all depends on the ratio of the components in them. For alloys it is written as a numerical interval.
Concept of temperature scale
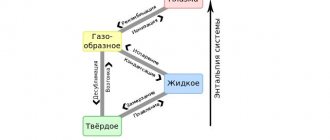
Some non-metallic objects also have similar properties. The most common is water. A temperature scale was developed regarding the properties of the liquid that occupies a dominant position on Earth. The reference points are the temperature of changes in the aggregative states of water:
- Transformations from liquid to solid and vice versa are taken to be zero degrees.
- Boiling (vapor formation inside a liquid) at normal atmospheric pressure (760 mm Hg) is taken to be 100 ⁰C.
Metal crystal lattices
In its ideal form, it is generally accepted that metals have a cubic lattice (real substances may have flaws). There are equal distances between molecules horizontally and vertically.
A solid substance is characterized by constancy:
- shapes, the object retains linear dimensions in different conditions;
- volume, the object does not change the amount of substance it occupies;
- mass, the amount of a substance expressed in grams (kilograms, tons);
- density, unit volume contains constant mass.
When transitioning into a liquid state, having reached a certain temperature, the crystal lattices are destroyed. Now we can’t talk about constancy of form. The liquid will take the form in which it is poured.
Read also: Groove for retaining rings
When evaporation occurs, only the mass of the substance remains constant. Gas will take up the entire volume that will be provided to it. Here we cannot say that density is a constant value.
When liquids combine, the following options are possible:
- Liquids completely dissolve in one another, as do water and alcohol. The concentration of substances will be the same throughout the entire volume.
- Liquids are stratified by density, the connection occurs only at the interface. It is only temporarily possible to obtain a mechanical mixture. Mix liquids with different properties. An example is oil and water.
The resulting substances, soluble in each other, when solidified, form crystal lattices of a new type. Define:
- Heliocentered crystal lattices are also called body-centered. In the middle there is a molecule of one substance, and four more molecules of another are located around it. It is customary to call such lattices loose, since the bonds between metal molecules in them are weaker.
- Face-centered crystal lattices form compounds in which the component molecules are located on the faces. Metallurgists call such crystalline alloys dense. In reality, the density of the alloy can be higher than that of each of the components included in the composition (alchemists of the Middle Ages were looking for options for alloys in which the density would correspond to the density of gold).
Melting point of metals
Different substances have different melting points. It is customary to divide metals into:
- Low-melting - it is enough to heat them to 600 ⁰C to obtain the substance in liquid form.
- Medium-melting metals melt in the temperature range 600…1600 ⁰С.
- Refractory are metals that can melt at temperatures above 1600 ⁰C.
The table shows low-melting metals in ascending order. Here you can see that the most unusual metal is mercury (Hg). Under normal conditions it is in a liquid state. This metal has the lowest melting point.
Table 1, melting and boiling points of fusible metals:
Table 2, melting and boiling points of medium-melting metals:
Table 3, melting and boiling points of refractory metals:
Various devices are used to carry out the smelting process. For example, blast furnaces are used to smelt iron. For melting non-ferrous metals, internal heating is carried out using high-frequency currents.
Molds made of non-metallic materials contain non-ferrous metals in a solid state. An alternating microwave magnetic field is created around them. As a result, the crystal lattices begin to loosen. The molecules of the substance begin to move, which causes heating within the entire mass.
If it is necessary to melt a small amount of low-melting metals, muffle furnaces are used. In them, the temperature rises to 1000...1200 ⁰С, which is enough for melting non-ferrous metals.
Ferrous metals are melted in convectors, open hearths and induction furnaces. The process involves the addition of alloying components that improve the quality of the metal.
It is most difficult to work with refractory metals. The problem is that you need to use materials that have a temperature higher than the melting point of the metal itself. The aircraft industry is currently considering the use of Titanium (Ti) as a structural material. At high flight speeds in the atmosphere, the skin heats up. Therefore, a replacement for aluminum and its alloys (AL) is needed.
Strength of metals
In addition to the ability to transition from a solid to a liquid state, one of the important properties of a material is its strength - the ability of a solid body to resist destruction and irreversible changes in shape. The main indicator of strength is the resistance that occurs when a pre-annealed workpiece breaks. The concept of strength does not apply to mercury because it is in a liquid state. The designation of strength is accepted in MPa - Mega Pascals.
There are the following strength groups of metals:
- Fragile. Their resistance does not exceed 50MPa. These include tin, lead, soft-alkaline metals
- Durable, 50−500 MPa. Copper, aluminum, iron, titanium. Materials of this group are the basis of many structural alloys.
- High strength, over 500 MPa. For example, molybdenum and tungsten.
Metal strength table
| Metal | Resistance, MPa |
| Copper | 200−250 |
| Silver | 150 |
| Tin | 27 |
| Gold | 120 |
| Lead | 18 |
| Zinc | 120−140 |
| Magnesium | 120−200 |
| Iron | 200−300 |
| Aluminum | 120 |
| Titanium | 580 |
Metal alloys
To design products from alloys, their properties are first studied. To study, the metals being studied are melted in small containers in different ratios to each other. Based on the results, graphs are built.
The lower axis represents the concentration of component A with component B. The vertical axis is temperature. Here the values of the maximum temperature are noted when all the metal is in a molten state.
When cooled, one of the components begins to form crystals. In a liquid state, eutectic is an ideal compound of metals in an alloy.
Metallurgists identify a special ratio of components at which the melting point is minimal. When making alloys, they try to select the amount of substances used in order to obtain a eutectoid alloy. Its mechanical properties are the best possible. Crystal lattices form ideal face-centered positions of atoms.
The crystallization process is studied by studying the hardening of samples upon cooling. They build special graphs where they observe how the cooling rate changes. Ready-made diagrams are available for different alloys. By marking the start and end points of crystallization, the composition of the alloy is determined.
Medium melting metals
Medium-melting metals begin to transform from solid to liquid at temperatures from 600°C to 1600°C. They are used to make slabs, reinforcements, blocks and other metal structures suitable for construction. This group of metals includes iron, copper, aluminum, and they are also part of many alloys. Copper is added to alloys of precious metals such as gold, silver, and platinum. 750 gold consists of 25% alloy metals, including copper, which gives it a reddish tint. The melting point of this material is 1084 °C. And aluminum begins to melt at a relatively low temperature of 660 degrees Celsius. This is a lightweight, ductile and inexpensive metal that does not oxidize or rust, therefore it is widely used in the manufacture of tableware. The melting point of iron is 1539 degrees. This is one of the most popular and affordable metals, its use is widespread in the construction and automotive industries. But due to the fact that iron is subject to corrosion, it must be additionally processed and covered with a protective layer of paint, drying oil, or prevent moisture from entering.
Read also: Get acetylene from ethylene
Soldering alloys
In practice, many people experience melting when soldering parts. If the surfaces of the materials to be joined are cleaned of contaminants and oxides, then they can be easily soldered with solders. It is customary to divide solders into hard and soft. Soft ones are most widespread:
- POS-15 - 278...282 °C;
- POS-25 - 258...262 °C;
- POS-33 - 245...249 °C;
- POS-40 - 236...241 °C;
- POS-61 - 181...185 °C;
- POS-90 - 217...222 °C.
They are produced for enterprises manufacturing various radio equipment.
Brazing alloys based on zinc, copper, silver and bismuth have a higher melting point:
- PSr-10 - 825...835 °C;
- PSr-12 - 780...790 °C;
- PSr-25 - 760...770 °C;
- PSr-45 - 715...721 °C;
- PSr-65 - 738...743 °C;
- PSr-70 - 778...783 °C;
- PMC-36 - 823...828 °C;
- PMC-42 - 830...837 °C;
- PMC-51 - 867...884 °C.
The use of hard solders allows you to obtain strong connections.
Attention! Wed means that silver is used in the solder. Such alloys have minimal electrical resistance.
Melting point of non-metals
Non-metallic materials can be presented in solid and liquid form. Inorganic substances are presented in table. 4.
Table 4, melting point of inorganic non-metals:
In practice, organic materials are of greatest interest to users: polyethylene, polypropylene, wax, paraffin and others. The melting points of some substances are shown in table. 5.
Table 5, melting temperature of polymer materials:
Attention! The glass transition temperature refers to the state at which a material becomes brittle.
Video: melting point of known metals.