The creation of metal structures from steel requires the selection of material in accordance with the technical specifications and taking into account the characteristics of steel grades. Determining such characteristics is possible by deciphering the alphanumeric code on the metal marking. However, such a mark cannot always be seen on workpieces; in this case, the determination of the metal grade is carried out using a laboratory method, including using methods based on testing mechanical properties.
Basic methods for determining steel grades
When working with metal, methods for determining the quality of steel are still used today by analyzing its mechanical and physical characteristics. Such methods, unlike laboratory ones, make it possible to approximately determine the qualitative characteristics of the sample, but for work, in particular for welding metal, this is quite enough. Such methods for studying steel grades include:
- When it comes to the strength of metal, the chip removal method is used. Its essence is to remove metal shavings using a chisel. Chips that crumble and form into small strips are characteristic of high-carbon steels. Long strips of ductile chips characterize the metal as steel with high ductility.
- The quenching method is used to approximate the carbon content of the workpiece. Using a saw blade, cuts are made on the workpiece before and after hardening. If in both cases the metal is easily sawn with a blade, it contains a small amount of carbon. If it is difficult to make cuts after processing, it means that the carbon concentration has increased.
- Determining the hardness of a metal by extracting a sheaf of sparks allows you to approximately determine which class of steel the metal belongs to. To do this, surface treatment of the workpiece sample is done on an emery wheel. The shape of the sparks, color, and intensity of the sheaf of sparks determines the hardness of the metal and the carbon content.
Under ordinary home conditions, it is almost impossible to accurately determine the brand and composition of the metal; for this purpose, laboratory tests are carried out, during which a detailed chemical and physical analysis of the metal is performed. The listed methods make it possible to determine only the general characteristics of steel based on its carbon content; the exact characteristics are not determined during such studies.
At the same time, even such a rapid analysis makes it possible to select samples for the manufacture of knives, cutters or parts of machine mechanisms with increased strength and wear resistance.
Steel quality
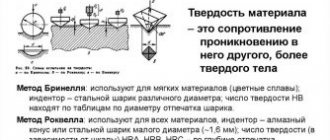
1. The quality of steel is determined by the content of harmful impurities..
The main harmful impurities are sulfur and phosphorus. Harmful impurities also include gases (nitrogen, oxygen, hydrogen).
Sulfur, a harmful impurity, enters steel mainly with the raw material - cast iron. sulfur is insoluble in iron; it forms the compound FeS with it - iron sulfide. upon interaction with iron, a eutectic (Fe + FeS) is formed with a melting point of 9880 C. Therefore, when steel blanks are heated for plastic deformation above 9000 C, the steel becomes brittle. During hot plastic deformation, the workpiece is destroyed. This phenomenon is called red brittleness. One way to reduce the influence of sulfur is to introduce manganese. The Mns compound melts at 16200 C; these inclusions are plastic and do not cause red brittleness.
The sulfur content in steels is allowed to be no more than 0.06%.
Phosphorus also enters steel mainly with the original cast iron, which is also used for steel smelting. Up to 1.2% phosphorus dissolves in ferrite, reducing its ductility. Phosphorus has a high tendency to segregate, so even with an insignificant average amount of phosphorus in the casting, areas rich in phosphorus can always form.
Phosphorus located near the boundaries increases the temperature of transition to a brittle state (cold brittleness). Therefore, phosphorus, like sulfur, is a harmful impurity; its content in carbon steel is allowed up to 0.050%.
Hidden impurities: This is the name given to the gases present in steel - nitrogen, oxygen, hydrogen - due to the difficulty of determining their quantity. Gases enter the steel during its smelting. In hard steel they can be present, either dissolving in ferrite or forming a chemical compound (nitrides, oxides). Gases can also be in a free state in various discontinuities.
Even in very small quantities, nitrogen, oxygen and hydrogen greatly impair the plastic properties of steel. Their content in steel is allowed 10-2 - 10-4%. As a result of evacuation of steel, their content decreases and properties improve.
There are two types of carbon tool steels: quality and high-quality.
High-quality carbon tool steels are marked with the letter “U” (carbon); the number following it (U7, U8, U10, etc.) shows the average carbon content in tenths of a percent.
High-quality steels are additionally marked with the letter “A” at the end (U10A).
Tool carbon steels: They have high hardness (60-65 HRC), strength and wear resistance and are used for the manufacture of various tools.
Carbon tool steels U8 (U8A), U10 (U10A), U11 (U11A), U12 (U12A) and U13 (U13A), due to the low stability of supercooled austenite, have low hardenability, and therefore these steels are used for small-sized tools.
For cutting tools (mills, countersinks, drills, spiral saws, scrapers, hand saws, files, razors, sharp surgical instruments, etc.), hypereutectoid steels (U10, U11, U12 and U13) are usually used, in which, after heat treatment, structure martensite and carbides.
Woodworking tools, chisels, punches, bits, screwdrivers, axes are made from U7 and U8 steels, which have a troostite structure after heat treatment.
Carbon steels in the initial (annealed) state have a granular pearlite structure, low hardness (HB 170-180) and are well machined by cutting. The hardening temperature of carbon tool steels U10-U13 should be 760-780 0 C, i.e. slightly higher than Ac1, but lower than Ast, so that, as a result of hardening, the steel obtains a martensitic structure and retains fine grains and insoluble particles of secondary cementite. Hardening is carried out in water or aqueous solutions of salts. Small tools made of U10-U12 steels are cooled in hot environments (step hardening) to reduce deformations.
Tempering is carried out at 150-1700 C to maintain high hardness (62-63 HRC).
U7 steel is hardened with heating above the Ac3 point (800-8200 C) and tempered at 275-325 0 C (48-58 HRC).
Carbon steels can be used as a cutting tool only for cutting materials at low speeds, since their high hardness is greatly reduced when heated above 190-200 0 C.
2. Iron-iron carbide phase diagram.
Steels containing from 0.8 to 2.14% C are called hypereutectoid.
At the beginning of heating, the hypereutectoid alloy has the structure of pearlite and secondary cementite.
When the temperature rises to 7270 C, the alloy simply heats up. In point 1, the eutectoid transformation occurs, pearlite transforms into austenite. From point 1 to point 2, the alloys have the structure of austenite + secondary cementite. As point 2 is approached, the carbon concentration in austenite increases according to the SE line.
At temperatures corresponding to the SE line (point 2), austenite turns out to be saturated with carbon, and with increasing temperature the alloy has the structure of only austenite. Up to point 3, no changes occur in the alloy, the temperature simply increases.
As the temperature increases at point 3, solid austenite begins to melt. The structure becomes liquid + austenite. Up to point 4, the alloy continues to melt.
At point 4, under the influence of high temperature, all austenite melts. The structure becomes liquid.
3. When heated above a temperature of 7270 C, the number of nuclei is always quite large and the initial austenite grain is small. The higher the heating rate, the smaller the austenite grain, since the rate of nucleation formation is higher than the rate of their growth.
With a further increase in temperature or an increase in the duration of exposure at a given temperature, collective recrystallization occurs and the grain increases. The growth of grains formed when heated to a given temperature naturally does not change upon subsequent cooling.
The ability of austenite grains to grain growth is not the same even for steels of the same grade composition due to the influence of their smelting conditions.
Based on their tendency to grain growth, two extreme types of steel are cast: hereditarily fine-grained and hereditarily coarse-grained.
In hereditarily fine-grained steel, when heated to high temperatures (1000-10500 C), the grain increases slightly, but with higher heating, rapid grain growth occurs. In hereditary coarse-grained steel, on the contrary, strong grain growth is observed even with slight overheating above 7270 C. The different tendency to grain growth is determined by the conditions of deoxidation of the steel and its composition.
The smaller the grain, the higher the strength (sв, st, s-1), ductility (d, y) and toughness (KCU, KCT), the lower the cold brittleness threshold (t50) and the lower the tendency to brittle fracture. By reducing the austenite grain size, it is possible to compensate for the negative influence of other hardening mechanisms on the cold brittleness threshold.
Alloying elements, especially carbide-forming (nitride-forming) elements, retard the growth of austenite grains. Ti, V, Nb, Zr, Al, and N act most strongly, forming carbides (nitrides) that are poorly soluble in austenite and serve as a barrier to grain growth. The larger the volume fraction of carbides (nitrides) and the higher their dispersion (smaller size), the finer the austenite grain. At the same time, insoluble carbides (natrides) have a nucleating effect on the formation of new austenite grains, which also leads to the production of finer grains. Manganese and phosphorus promote austenite grain growth.
All methods that cause refinement of austenite grains - microalloying (V, Ti, Nb, etc.), high heating rates, etc. - increase the structural strength of steel.
They strive to obtain coarse grains only in electrical (transformer) steels in order to improve their magnetic properties.
- Back
- Forward
Marking of steels and their properties
Much more information about the metal can be provided by the markings applied to the samples or the code indicated in the accompanying documents. In the countries of the former USSR, markings for metal for domestic use are indicated in accordance with national state standards developed on the basis of the steel grade of the former USSR. For foreign customers, markings are indicated in accordance with international standards.
For domestic samples, the steel designation is indicated in the first letter code with the signs “St” - steel. The first number after the letter designation indicates the amount of carbon contained in the metal, followed by the codes of alloying components and additives. Letter designations indicate the content of individual additives that significantly affect the quality of the metal, for example, chromium, copper or aluminum.
The description may also indicate separately the purpose of the steel:
- Structural;
- Instrumental;
- Mechanical engineering;
- High-speed;
- Low alloy;
- Wear-resistant;
- Magnetic;
- Stainless;
- Heat resistant.
More detailed information about the chemical and physical properties of such steels can be found in collections of characteristics of grade steels. The reference standards indicated in the reference book provide ideas about the properties of the metal and the possibilities of its application.
Designation of steels with alloying elements
As mentioned above, the classification of steels with alloying elements includes several categories. The marking of alloy steels is compiled according to certain rules, knowledge of which allows one to quite simply determine the category of a particular alloy and the main area of its application. In the initial part of the names of such brands there are numbers (two or one) indicating the carbon content. Two numbers indicate its average content in the alloy in hundredths of a percent, and one – in tenths. There are also steels that do not have numbers at the beginning of the brand name. This means that the carbon content in these alloys is within 1%.
Example of alloy steel marking
The letters that can be seen behind the first digits of the brand name indicate what the alloy is made of. The letters that give information about a particular element in its composition may or may not be followed by numbers. If there is a number, then it determines (in whole percentages) the average content of the element indicated by the letter in the alloy, and if there is no number, it means that this element is contained in the range from 1 to 1.5%.
At the end of the marking of certain types of steel there may be the letter “A”. This suggests that this is high quality steel. These grades may include carbon steels and alloys with alloying additives in their composition. According to the classification, this category of steels includes those in which sulfur and phosphorus amount to no more than 0.03%.
Features of the material
Any steel, as is known, is an alloy of iron and carbon, to which various alloying elements can be added. The division of steels into low-, medium- and high-carbon types depends on the amount of carbon present in their composition. This element, which has a serious impact on the characteristics of the finished alloy, can be contained in steels from 0.02 to 2.14%. In steels classified as high-carbon, the amount of this element in the composition starts at 0.6%.
Steel grades and the proportion of various elements in their composition
One of the distinctive features that high-carbon steels have is that products made from them are difficult to weld; welding leads to cracks appearing in the weld zone. This is explained by the fact that such materials, having a certain chemical composition, have a tendency to form hardened zones in those places where the metal is exposed to thermal effects.
Due to this feature of high-carbon steels, welding products made from them should only be done using electrodes with low thermal power. The welding arc used to connect products made of high-carbon steels must be of the recovery type. The use of an oxidizing arc in such cases will lead to the fact that carbon will be burned out of the steel composition, and, as a result, the metal in the weld area will become more porous. Meanwhile, such a negative effect can be avoided if the products to be joined are preheated to a temperature of 200–2500.
Violation of the technological features of welding high-carbon steels leads to weld defects
Popular steel grades and their applications
Steels are usually called alloys of iron and carbon containing up to 2.14% carbon. Depending on the chemical composition, carbon steels (GOST 380-71, GOST 1050-75) and alloy steels (GOST 4543-71, GOST 5632-72, GOST 14959-79) are distinguished.
Basic steel production standards:
- carbon steel of ordinary quality (GOST 380-88);
- structural steel (GOST 1414-75);
- high-quality carbon structural steel (GOST 1050-88);
- tool carbon steel (GOST 1435-90);
- alloyed structural steel (GOST 4543-71);
- high-quality low-carbon steel (GOST 9045-80);
- low-alloy structural steel (19281-89).
- high-quality calibrated steel (GOST 1051-73);
- bearing steel (GOST 801-78)
- low-alloy reinforcing steel (GOST 5781-82);
- alloy structural steel (GOST 4543-71);
- alloy tool steel (GOST 5950-73);
- high-alloy steels and alloys that are corrosion-resistant, heat-resistant and heat-resistant (GOST 5632-72);
- high-quality alloyed structural steel for special purposes (GOST 11268-76) and some others.
Chemical composition of carbon structural steels of ordinary quality
The most popular steel grades
St 0 – non-critical building structures, gaskets, washers, casings. St. 1 – lightly loaded parts of metal structures. Weldability is good. St. 2 – parts of metal structures – frames, axles, keys, rollers, cemented parts. Weldability is good. St 3 - parts of metal structures, trolley frames, crane hooks, cemented parts with high surface hardness and low core strength. St 4 – shafts, rods, hooks, axles, bolts (low strength requirements). St 5 – sprockets, gears, shafts, axles (increased strength requirements). St 6 – spindles, couplings, shafts (high strength). 08KP, 10 – parts manufactured by cold stamping and cold heading, fasteners, cemented parts. 15, 20 – lightly loaded parts (pins, stops, axles, gears) subject to wear. 30, 35 – traverses, rods, levers, disks, sprockets, shafts. 40, 45 – high-strength parts subjected to heat treatment (crankshafts, connecting rods, ring gears, ratchets, couplings, plungers). 50, 55 – gear wheels, rolling rolls, spindles, bandages, lightly loaded springs and springs. 60 – parts with high strength properties (rolling rolls, spring rings, clutch springs and discs, shock absorber springs). 09G2S – for steam boilers, apparatus and containers operating under pressure at temperatures -70...+450*C, for critical sheet welded structures, in chemical and petroleum engineering, shipbuilding. 10HSND – for welded structures and shaped profiles in shipbuilding, carriage building, chemical engineering. 15HSND – parts of carriages, construction piles, shipbuilding profiles. Has increased corrosion resistance. 40X – parts operating at medium speeds and medium pressures (gears, splined shafts). 18ХГТ - parts operating at high speeds under high pressures and shock loads (gears, jaw couplings, bushings). 30KhGSA – high-strength parts, critical welded structures. 08Х18Н10 – parts operating in aggressive environments at elevated temperatures. 08Х18Н10Т – for welded structures in various industries. 65...80, 65G, 50HFA, 60S2A - springs, springs. U8A – knurling rollers, countersinks, chisels. U10A – taps, needle files, smooth gauges. HGS – cold rolling rolls, dies, punches. HVG – measuring, cutting instrument. Х12, Х12ВМ – for cold stamps. 4ХС – hot heading dies. A12, A20 – complex-profile small parts (gears, pins, rings, screws). A30, A40G – difficult-to-machine parts operating under high loads. ШХ15 – balls with a diameter of up to 150 mm, rollers with a diameter of up to 23 mm, plungers.
High-quality and high-quality steel
27. Structural steel is steel that is used for the manufacture of various parts, mechanisms and structures in mechanical engineering and construction and has certain mechanical, physical and chemical properties. Structural steels are divided into several subgroups.
Quality of structural carbon steels
The quality of structural carbon steels is determined by the presence of harmful impurities of phosphorus (P) and sulfur (S) in the steel. Phosphorus - gives steel cold brittleness (brittleness). Sulfur, the most harmful impurity, makes steel red-brittle. Content of harmful impurities in steel:
· Ordinary quality - P and S - up to 0.05% (marking St ).
· High-quality - P and S - up to 0.035% (marked Steel ).
· High quality - P and S - up to 0.025% (marking A at the end of the brand).
· Extra high quality - P and S - up to 0.015% (marking Ш at the end of the brand).
Structural carbon steel of ordinary quality
They are widely used in construction and mechanical engineering, as they are the cheapest, most technologically advanced, and have the necessary properties for the manufacture of structures for mass use. Basically, these steels are used in the hot-rolled state without additional heat treatment with a ferrite-pearlite structure. Depending on the subsequent purpose, structural carbon steels of ordinary quality are divided into three groups: A, B, C.
Steel group A
Supplied with certain regulated mechanical properties. Their chemical composition is not regulated. These steels are used in structures whose components are not subjected to hot processing - forging, hot stamping, heat treatment, etc. In this regard, the mechanical properties of hot-rolled steel are preserved.
Group B steel
They are supplied with a certain regulated chemical composition, without guarantee of mechanical properties. These steels are used in products subjected to hot processing, the technology of which depends on their chemical composition, and the final mechanical properties are determined by the processing itself.
Steel group B
Supplied with regulated mechanical properties and chemical composition. These steels are used for the manufacture of welded structures. Their weldability is determined by the chemical composition, and the mechanical properties outside the welding zone are determined in the delivery state. Such steels are used for more critical parts.
According to the degree of deoxidation
The degree of deoxidation is determined by the silicon (Si) content of the steel. According to the degree of deoxidation, carbon steels of ordinary quality are divided into:
calm (SP) - 0.12-0.3% (Si)
semi-quiet (PS) - 0.07-0.12% (Si)
· boiling (KP) - no more than 0.07% (Si)
Marking
The main grades of structural carbon steels of ordinary quality:
St1kp2; BSt2ps; VSt3Gps; St4-2; ... VSt6sp3.
· The letter in front of the mark indicates the steel group. A steel is not designated by a letter.
· St - shows that the steel is of ordinary quality.
· The first digit is the GOST number (from 0 to 6).
· The letter G after the first digit means increased content of manganese (Mn) - (serves to increase the hardenability of steel).
· sp; ps; kp - degree of deoxidation of steel (For steel group A, the absence of a designation implies “sp”).
· The second digit is the steel category number (from 1 to 6 - basic mechanical properties). Steel of the 1st category is not indicated by a number.
· The dash between the numbers indicates that the customer did not make any requirements for the degree of steel deoxidation.
Application
· St1; St2 - wire, nails, rivets.
· St3; St4 - fasteners, shaped steel.
· St5; St6 - lightly loaded shafts, axles.
Marking
· Steel – the word “Steel” indicates that this carbon steel is of high quality. (Currently the word “Steel” is not written, only the index and subsequent letters are indicated)
· The number indicates the carbon content (C) in the steel in hundredths of a percent.
Application
Low-carbon steel grades Stal08, Stal08KP, Stal08PS are mild steels, most often used in the annealed state for the manufacture of parts by cold stamping - deep drawing. steel grades Steel10, Steel15, Steel20, Steel25 are usually used as cemented ones, and high-carbon Steel60 ... Steel85 - for the manufacture of springs, springs, high-strength wire and other products with high elasticity and wear resistance.
Steel30 ... Steel50 and similar steels with a high manganese content Steel30G, Steel40G, Steel50G are used for the manufacture of a wide variety of machine parts.
Marking
automatic steel there is always the letter A.
Marking
· Two numbers at the beginning of the marking indicate structural steels (one number indicates tool steels). This is the carbon content in steel in hundredths of a percent.
· A letter without a number is a specific alloying element with a content in steel of less than 1%. (A-nitrogen, P-boron, F-vanadium, G-manganese, D-copper, K-cobalt, M-molybdenum, N-nickel, C -silicon, X-chromium, P-phosphorus, H-rare earth metals, B-tungsten, T-titanium, Yu-aluminum, B-niobium)
· The letter and number after it are a specific alloying element with a percentage content (number).
· The letter A at the end of the marking indicates high-quality steel.
· For example, 38Х2Н5МА is a medium-alloyed high-quality chromium-nickel structural steel. Chemical composition: carbon - about 0.38%; chromium - about 2%; nickel - about 5%; molybdenum - about 1%.
Superhard materials
For the manufacture of various cutting tools, three types of superhard materials (SHM) are currently used in various industries, including mechanical engineering: natural diamonds, polycrystalline synthetic diamonds and composites based on boron nitrite (CBN).
Natural and synthetic diamonds have such unique properties as the highest hardness (HV 10,000 kgf/mm 2), they have very low: linear expansion coefficient and friction coefficient; high: thermal conductivity, adhesive resistance and wear resistance. The disadvantages of diamonds are low bending strength, brittleness and solubility in iron at relatively low temperatures (+750 °C), which prevents their use for processing iron-carbon steels and alloys at high cutting speeds, as well as during intermittent cutting and vibration. Natural diamonds are used in the form of crystals fixed in the metal body of the cutter. Synthetic diamonds of the ASB (balas) and ASPC (carbonado) brands are similar in structure to natural diamonds. They have a polycrystalline structure and have higher strength characteristics.
Natural and synthetic diamonds are widely used in the processing of copper, aluminum and magnesium alloys, precious metals (gold, silver), titanium and its alloys, non-metallic materials (plastics, textolite, fiberglass), as well as hard alloys and ceramics.
Synthetic diamonds have a number of advantages over natural diamonds due to their higher strength and dynamic characteristics. They can be used not only for turning, but also for milling.
The composite is a super-hard material based on cubic boron nitride, used for the manufacture of blade cutting tools. In terms of hardness, the composite approaches diamond, significantly exceeds it in heat resistance, and is more inert to ferrous metals. This determines its main area of application - the processing of hardened steels and cast irons. The industry produces the following main brands of STM: composite 01 (elbor - R), composite 02 (belbor), composite 05 and 05I and composite 09 (PTNB - NK).
Composites 01 and 02 have high hardness (HV 750 kgf/mm2), but low bending strength (40–50 kg/mm2). Their main area of application is fine and fine non-impact turning of parts made of hardened steels with a hardness of HRC 55–70, cast irons of any hardness and hard alloys of grades VK 15, VK 20 and VK 25 (HP^ 88–90), with a feed of up to 0.15 mm /rev and cutting depth 0.05-0.5 mm. Composites 01 and 02 can also be used for milling hardened steels and cast irons, despite the presence of shock loads, which is explained by more favorable dynamics of milling. Composite 05 occupies an intermediate position in hardness between composite 01 and composite 10, and its strength is approximately the same as that of composite 01. Composites 09 and 10 have approximately the same bending strength (70-100 kgf/mm2).
Copper alloys
Copper is one of the metals known since ancient times. Man's early acquaintance with copper was facilitated by the fact that it occurs in nature in a free state in the form of nuggets, which sometimes reach significant sizes. Currently, copper is widely used in electrical engineering, in the construction of power lines, for the manufacture of telegraph and telephone communication equipment, radio and television equipment. Wires, cables, busbars and other conductive products are made from copper. Copper has high electrical and thermal conductivity, strength, toughness and corrosion resistance. Its physical properties are determined by its structure. It has a cubic gra-non-centered spatial lattice. Its melting point is +1083 °C, boiling point is +2360 °C. The average tensile strength depends on the type of treatment and ranges from 220 to 420 MPa (22–45 kgf/mm2), relative elongation – 4–60%, hardness – 35–130 HB, density – 8.94 g/cm3. Having remarkable properties, copper at the same time as a structural material does not satisfy the requirements of mechanical engineering, so it is alloyed, i.e., metals such as zinc, tin, aluminum, nickel and others are added to alloys, thereby improving its mechanical and technological properties . In its pure form, copper is used to a limited extent; its alloys are used more widely. According to their chemical composition, copper alloys are divided into brass, bronze and copper-nickel, and according to their technological purpose - into wrought, used for the production of semi-finished products (wire, sheet, strip, profile), and foundry, used for casting parts.
Brasses are alloys of copper with zinc and other components. Brasses containing, in addition to zinc, other alloying elements are called complex, or special, and are named according to the alloying components introduced, in addition to zinc. For example: tompak L90 is brass containing 90% copper, the rest is zinc; aluminum brass LA77–2 - 77% copper, 2% aluminum, the rest is zinc, etc. Compared to copper, brasses have great strength, corrosion resistance and elasticity. They are processed by casting, pressing and cutting. Semi-finished products are made from them (sheets, tapes, strips, pipes of condensers and heat exchangers, wire, stampings, shut-off valves - taps, valves, medals and badges, artistic products, musical instruments, bellows, bearings).
Bronzes are copper-based alloys in which tin, aluminum, beryllium, silicon, lead, chromium and other elements are used as additives. Bronzes are divided into tin-free (BrA9Mts2L, etc.), tin (BrO3ts12S5, etc.), aluminum (BrA5, BrA7, etc.), silicon (BrKN1–3, BrKMts3–1), manganese (BrMts5), beryllium bronzes (BrB2, BrBNT1,7, etc.). Bronze is used for the production of shut-off valves (taps, valves), various parts operating in water, oil, steam, mildly aggressive environments, and sea water.
Aluminum alloys
The name "aluminum" comes from the Latin word alumen - so 500 years BC. e. called aluminum alum, which was used for etching when dyeing fabrics and tanning leather.
In terms of abundance in nature, aluminum ranks third after oxygen and silicon and first among metals. In terms of use in technology, it ranks second after iron. Aluminum is not found in free form; it is obtained from minerals - bauxite, nepheline and alunite, while alumina is first produced, and then aluminum is obtained from alumina by electrolysis. The mechanical properties of aluminum are low: tensile strength – 50–90 MPa (5–9 kgf/mm2), relative elongation – 25–45%, hardness – 13–28 HB.
Aluminum welds well, but is difficult to machine, has a large linear shrinkage - 1.8% In its pure form, aluminum is rarely used; its alloys with copper, magnesium, silicon, iron, etc. are widely used. Aluminum and its alloys are necessary for aviation and mechanical engineering, power lines, metro and railway rolling stock.
Aluminum alloys are divided into cast and wrought. Cast aluminum alloys are produced in ingots - refined and unrefined.
Alloys with the letter “P” in their brand designations are intended for the manufacture of food utensils. The mechanical properties of alloys depend on their chemical composition and production methods. The chemical composition of the main components included in the alloy can be determined by grade. For example, AK12 alloy contains 12% silicon, the rest is aluminum; AK7M2P – 7% silicon, 2% copper, the rest is aluminum. The most widely used aluminum-silicon alloy in various industries is silumin, which is produced in four grades: SIL-00,
STR-0, STR-1 and STR-2. In addition to aluminum (base) and silicon (10–13%), this alloy includes: iron – 0.2–0.7%, manganese – 0.05–0.5%, calcium – 0.7–0.2 %, titanium - 0.05-0.2%, copper - 0.03% and zinc - 0.08%. Various parts for cars, tractors, and passenger cars are made from silumins. Aluminum wrought alloys in ingots, intended for pressure processing and for shearing in the production of other aluminum alloys, are standardized by certain standards. Alloys for forming consist of aluminum (base), alloying elements (copper - 5%, magnesium - 0.1-2.8%, manganese - 0.1-0.7%, silicon - 0.8-2.2 %, zinc - 2–6.5% and a small amount of other impurities). The grades of these alloys are: VD1, AVD1, AVD1–1, AKM; semi-finished products are made from aluminum alloys - sheets, strips, strips, slabs, ingots, slabs.
In addition, non-ferrous metallurgy produces aluminum anti-friction alloys used for the manufacture of monometallic and bimetallic bearings by casting. Depending on the chemical composition, the standard provides for the following grades of these alloys: AO3–7, AO9–2, AO6–1, AO9–1, AO20–1, AMST. The standard also defines the operating conditions for products made from these alloys: load from 19.5 to 39.2 MN/m2 (200–400 kgf/cm2), temperature from 100 to 120 °C, hardness from 200 to 320 HB.
Titanium alloys
Titanium is a silver-white metal. It is one of the most common elements in nature. Among other elements in terms of abundance in the earth’s crust (0.61%), it ranks tenth. Titanium is light (its density is 4.5 g/cm3), refractory (melting point 1665 °C), very durable and ductile. A persistent oxide film forms on its surface, due to which it resists corrosion well in fresh and sea water, as well as in some acids. At temperatures up to 882 °C it has a hexagonal densely packed lattice, at higher temperatures it has a body-centered cube. The mechanical properties of titanium sheets depend on the chemical composition and method of heat treatment. Its tensile strength is 300–1200 MPa (30–120 KGS/mm2), relative elongation is 4–10%. The harmful impurities of titanium are nitrogen, carbon, oxygen and hydrogen. They reduce its ductility and weldability, increase hardness and strength, and worsen corrosion resistance.
At temperatures above 500 °C, titanium and its alloys easily oxidize, absorbing hydrogen, which causes embrittlement (hydrogen embrittlement). When heated above 800 °C, titanium energetically absorbs oxygen, nitrogen and hydrogen; this ability is used in metallurgy to deoxidize steel. It serves as an alloying element for other non-ferrous metals and for steel.
Due to their remarkable properties, titanium and its alloys are widely used in aircraft, rocket and shipbuilding. Semi-finished products are made from titanium and its alloys: sheets, pipes, rods and wire. The main industrial materials for titanium production are ilmenite, rutile, perovskite and sphene (titanite). The technology for producing titanium is complex, labor-intensive and time-consuming: first, titanium sponge is produced, and then malleable titanium is produced from it by melting in vacuum furnaces.
Titanium sponge, produced by the magnesium-thermal method, serves as the starting material for the production of titanium alloys and other purposes. Depending on the chemical composition and mechanical properties, the following grades of titanium sponge are established as standard: TG-90, TG-100, TG-110, TG-120, TG-130. In the designation of brands, the letters “TG” mean spongy titanium, “Tv” means hard, and the numbers mean Brinell hardness. Titanium sponge contains impurities: iron - up to 0.2%, silicon - up to 0.04%, nickel - up to 0.05%, carbon - up to 0.05%, chlorine - up to 0.12%, nitrogen - up to 0 .04%, oxygen – up to 0.1%. Titanium and titanium alloys processed by pressure are intended for the manufacture of various semi-finished products (sheets, pipes, rods, wire). Depending on the chemical composition, the standard provides for the following grades: VT1-00, VT1-0, OT4-0, OT4-1, OT4, VT5, VT5-1, VT6, VT20, VT22, PT-7M, PT-7V, PT –1 m. Main components: aluminum – 0.2–0.7%, manganese – 0.2–2%, molybdenum – 0.5–5.5%, vanadium – 0.8–5.5%, zirconium – 0.8–3%, chromium – 0.5–2.3%, tin – 2–3%, silicon – 0.15–0.40%, iron – 0.2–1.5%. Iron, silicon and zirconium, depending on the brand of the alloy, can be the main components or impurities.
Zinc alloys
An alloy of zinc and copper - brass - was known to the ancient Greeks and Egyptians. But zinc smelting on an industrial scale began only in the 17th century.
Zinc is a light gray-bluish metal, brittle at room temperature and at 200 °C, and becomes ductile when heated to 100–150 °C.
In accordance with the standard, zinc is produced and supplied in the form of pigs and blocks weighing up to 25 kg. The standard also establishes grades of zinc and their areas of application: TsV00 (zinc content - 99.997%) - for scientific purposes, the production of chemical reagents, the manufacture of products for the electrical industry; CVO (zinc – 99.995%) – for the printing and automotive industries; TsV1, TsV (zinc – 99.99%) – for the production of injection moldings intended for the manufacture of critical parts, for the production of zinc oxide, zinc powder and pure reagents; TsOA (zinc 99.98%), TsO (zinc 99.975%) - for the production of zinc sheets, pressure-processed zinc alloys, whitewash, alloys, for hot-dip and galvanizing; Ts1S, Ts1, Ts2S, Ts2, Ts3S, Ts3 - for various purposes.
Zinc alloys are widely used in industry: brass, zinc bronze, alloys for coating various steel products, manufacturing galvanic elements, printing, etc. Zinc alloys in ingots for casting are standardized by the standard. These alloys are used in automobile and instrument making, as well as in other industries. The standard establishes the grades of alloys, their chemical composition, and defines the products made from them:
1) TsAM4–10 – especially critical parts;
2) TsAM4–1 – critical parts;
3) TsAM4–1V – non-critical parts;
4) TsA4O – critical parts with stable dimensions;
5) TsA4 – non-critical parts with stable dimensions.
Zinc antifriction alloys intended for the production of monometallic and bimetallic products, as well as semi-finished products, using casting and pressure processing methods are standardized by the standard. The mechanical properties of alloys depend on their chemical composition: tensile strength ?B
= 250–350 MPa (25–35 KGS/mm2), relative elongation
?
= 0.4–10%, hardness – 85–100 HB. The standard establishes the grades of these alloys, their areas of application and operating conditions: TsAM9–1.5L – casting of monometallic liners, bushings and sliders; permissible: load – 10 MPa (100 kgf/cm2), sliding speed – 8 m/s, temperature 80 °C; if bimetallic parts are produced by casting in the presence of a metal frame, then the load, sliding speed and temperature can be increased to 20 MPa (200 KGS/cm2), 10 m/s and 100 °C, respectively: TsAM9–1.5 - obtaining bimetallic tapes (zinc alloy with steel and duralumin) by rolling method, the tape is intended for the manufacture of liners by stamping; permissible: load – up to 25 MPa (250 kgf/cm2), sliding speed – up to 15 m/s, temperature 100 °C; AM10–5L – casting of bearings and bushings, permissible: load – 10 MPa (100 KGS/cm2), sliding speed – 8 m/s, temperature 80 °C.
32. CARBON GRAPHITE MATERIALS , tech. materials based on nature. or synthetic graphite They are characterized by high heat resistance (up to 3700 °C at pressures up to 20 GPa), high strength at higher temperatures. t-rah, oxidize, resistance to air, steam-air and aggressive non-oxidize. environments; Some carbon-graphite materials also have a high (up to 800 GPa) elastic modulus.
Carbon-graphite materials usually include coal coke, petroleum coke, decomp. types of graphite, glassy carbon, carbon-carbon materials, carbon fibers, carbon black (soot).
High price
The high cost of CM is due to the high knowledge intensity of production, the need to use special expensive equipment and raw materials, and therefore the developed industrial production and scientific base of the country.
Anisotropy of properties
Anisotropy is the dependence of CM properties on the choice of measurement direction. For example, the modulus of elasticity of unidirectional carbon fiber along the fibers is 10-15 times higher than in the transverse direction.
To compensate for anisotropy, the safety factor is increased, which can offset the advantage of CM in specific strength. An example of this is the experience of using CM in the manufacture of the vertical tail of the MiG-29 fighter. Due to the anisotropy of the used CM, the vertical tail was designed with a safety factor that was a multiple of the standard aviation coefficient of 1.5, which ultimately led to the fact that the composite vertical tail of the Mig-29 turned out to be equal in weight to the structure of the classic vertical tail made of duralumin .
Low impact strength
Low impact strength also causes the need to increase the safety factor. In addition, low impact strength causes high damage to CM products and a high probability of hidden defects that can only be detected by instrumental testing methods.
High specific volume
The high specific volume is a significant drawback when using CM in areas with strict restrictions on the occupied volume. This applies, for example, to the field of supersonic aviation, where even a slight increase in the volume of the aircraft leads to a significant increase in wave aerodynamic drag.
Hygroscopicity
Composite materials are hygroscopic, that is, they tend to absorb moisture, which is due to the discontinuity of the internal structure of the CM. During long-term operation and repeated temperature transitions through 0 Celsius, water penetrating into the structure of the CM destroys the CM product from the inside (the effect is similar in nature to the destruction of highways in the off-season). Thus, one of the possible causes of the American Airlines Flight 587 plane crash, in which the composite keel was torn off the fuselage, is said to be the destruction of the structure of the composite keel from water that periodically froze in it. Similar examples of separation of the composite fin from the fuselage also occurred in Russia.[2]
CM can also absorb other liquids with high penetrating ability, for example, aviation kerosene.
Toxicity
During operation, CMs can emit fumes that are often toxic. If CM is used to make products that will be located in close proximity to humans (the composite fuselage of the Boeing 787 Dreamliner may serve as such an example), then additional research into the effects of CM components on humans is required to approve the materials used in the manufacture of CM.
High-quality and high-quality steel
These steels are characterized by a lower content of harmful impurities (0.03 S and P) than ordinary quality steels. They are supplied in rental form. Forgings of other semi-finished products with guaranteed chemical composition and fur. Holy you. They are marked with two-digit numbers 05, 08, 10, 15, 20,...,85, indicating the average carbon content in hundredths of % (GOST 1050-88). Calm steels are marked without an index, semi-quiet steels - ps, boiling steels - kp. If the steel is high-quality, then the letter A is placed at the end (Steel45A). The content of S and P is not more than 0.02%. High-quality steels find many applications in technology, because... depending on the content. With and heat treatment have a variety of fur. and technological Holy you. Steels 05, 08, 10 – low-strength, highly plastic, approx. for cold stamping of various products. Without hot-rolled steel, they are used for washers, gaskets, casings, etc. Steels 15, 20, 25 - case hardened, for small parts: cams, pushers, lightly loaded gears.
25. Tool steels and hard alloys.
Intrum steels are carbon and alloy steels with high hardness 63-65 HRC, red resistance (heat resistance) - the ability not to decrease their hardness with increasing temperature. Typically these are steels consisting of martensite and excess carbides after quenching and low tempering. They are divided into:
1.alloyed and carbon steels with a small content of alloying elements and do not have heat resistance, work up to 200 degrees. U7…U13,Х,ХВГ (G-manganese),В1,ХГ2М.
2. alloy steels containing up to 18% alloying elements. Heat resistance 400-500 degrees. Х12,Х12М,Х12М2, 5ХНВ,5ХГМ,5ХНТ, etc.
3. heat-resistant, high-alloy steels of the ledeburite class, containing V, Cr, Co, Mo, etc. Heat resistance 600-650 degrees. High-speed ones are marked “P”. R18, R9K5, R6M5K5, 4K5V2FS,
4K8V2,3K7V7. All
High speed steels contain 4% Cr, but it is not indicated.
By purpose:
1.for cutting tools (must have high hardness of the cutting edge, all 3 types are used for manufacturing) 2.for cold forming die 3.for hot forming die 4.for measuring tools and high strength parts that require wear resistance and stability. They are processed by TMT, which consists of hardening and subsequent repeated tempering, which increases the hardness. Sometimes it undergoes additional processing: low-temperature nitriding, cyanidation, high-temperature steam treatment.
Solid solutions are metal-ceramic materials produced by the powder method. Hardness 87-92 HRC. Alloys used in industry are divided into:
1. tungsten VK8, VK3, VK6
2. tungsten, titanium, cobalt oxide. T15K6, T30K4, T15K10
3. carbides Ti, Co, V, Ta. TT8K6.
At the end “B” (coarse powder), “M” fine.
26.Designation of alloy grades. steels Their classification.
1. according to equilibrium structure: 1.1. hypoeutectoid. steel (excessive F in the structure);
1.2.eutectoid (P); 1.3.hypoeutect. (excess secondary alloyed carbides); 1.4. ledeburite (primary carbides released during crystallization from liquid steel - crystals). According to its structure, L should be classified as cast iron. In legir. steels with lower content. carbon is released L. In cast iron L is not deformed, it is very brittle, alloyed. In steels it is deformed, so steels with L are more brittle. In some legir. carbon steels m.b. >2.14%. 2. by structure after cooled. in air: 2.1. pearlite; 2.2. martensitic; 2.3. austenitic. This classification was proposed by the Frenchman Guillet. The German Ober Dörfer proposed a different classification. According to it, the structure could be: P; M; A; F; carbide (L); benite; F-M; A-F; A-M.
3. composition: 3.1. nickel; 3.2. chromium; 3.3. chromium-nickel; 3.4. chromium-nickel-molybdenum, etc. 4. for intended purposes: 4.1. structural; 4.2. instrumental; 4.3. steels and alloys with special properties (stainless, heat-resistant, heat-resistant, electrical, etc.). If the steel is alloyed. elements >50%, then it is an Fe-based alloy. Presence of legir. elements are specified in the steel grade. For this purpose, conventional designations are adopted: these are either the first letters of the name of the element, or the most sounding letters, or neither. H-Ni; X-Cr; B-tungsten; M-molybdenum; T-titanium; D-Cu; F–vanadium; E–selenium; Ch-rare earth elements; R-boron; K-cobalt; A-nitrogen; Yu-Al; B-niobium; G-Mg; C-silicon. In addition, to designate some groups approx. other symbols, and these symbols are placed in front of the brand: E-el.-technical. steel for post magnets; F-stainless steel Perlite class; I am stainless steel Aust class; R-high-speed become; U-carbon tool steels; Electrical-technical become; EI-experimental research; EP-experimental-trial. Example: 12Х18Н10Т; 30ХГСА.
27. Structural steel is steel that is used for the manufacture of various parts, mechanisms and structures in mechanical engineering and construction and has certain mechanical, physical and chemical properties. Structural steels are divided into several subgroups.
1Next ⇒
Recommended pages: