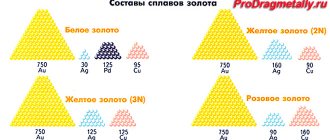
Characteristics of metals
Bronze is an alloy of copper and tin, silicon, beryllium, aluminum, lead and other elements. However, only tin is often used to obtain high-quality bronze. There are also alloys that use nickel and/or zinc. They are called spiater and are a cheap analogue of bronze.
Depending on what metal is present in the alloy, bronze is distinguished:
- tin;
- beryllium;
- aluminum;
- silicon
Thanks to this variety, the material is divided into two large groups - tin and tin-free. Previously, arsenic bronze also existed, but it was not widely used.
Brass is also an alloy, but here the main element is zinc combined with copper , to which nickel, tin, lead, manganese, iron or other elements may sometimes be added. Already in Ancient Rome, methods for producing this alloy were known. The Romans learned to smelt copper with zinc ore. It was only in 1781 that zinc in its pure form began to be used in England to produce brass. In the nineteenth century, due to its special color, this metal began to be used as counterfeit gold, and this quickly spread to many countries.
Currently, such an alloy is used to produce steel-brass bimetal. It is resistant to corrosion and abrasion, and is also quite flexible. In addition to the fact that brass is used in industry, its variety, called tombak, is quite often used for the manufacture of fittings, artistic products and insignia.
This is interesting: Brass - composition, grades, characteristics of the alloy
Comparison criteria
Despite the fact that in metallurgy there are clear criteria for distinguishing these two alloys, in real life an uninformed person is unlikely to accurately identify them.
Compound
Clear differences between metals can be traced only if the alloys do not contain impurities. However, now there are a large number of their varieties, which makes identification difficult. Brass is designated by the letter “L”; subsequent symbols in the marking indicate the presence of the main elements and the average copper content as a percentage. For example, L70 means 70% Cu content, and LAZ60-1-1 consists of 60% copper, 1% aluminum and 1% iron.
In a brass alloy, copper is combined with zinc, which gives the metal ductility and a low degree of wear resistance. This is the main additional element, but brass can be two-component or multi-component. There are different types of it.
- Deformable alloys. They are used in the production of machine parts, pipes, and springs.
- Foundry. They are used to make bearings, fittings, and devices designed to operate in conditions of elevated temperatures and aggressive environments.
- Jewelry brass. It is used to make jewelry, medals, decorative elements, and art products.
In bronze, copper is alloyed with tin, which makes the metal stronger, stronger and more durable. But sometimes aluminum, beryllium or magnesium are used instead. There are also several types of bronze.
- Tin bronze, also known as “bell” bronze. The main alloying element is tin. The alloy has good corrosion resistance and high anti-friction properties.
- Tin-free alloys . They use other components (aluminum, lead, beryllium, silicon and the like). These alloys are much softer and more ductile. The color of the material depends on the components it contains. For example, aluminum bronze has a distinctive golden-yellow color, which is why it has long been used as a substitute for gold in the production of coins and jewelry.
The most famous of the tin-free alloys is constantan. This heat-stable metal with high resistivity contains copper (about 59%) with the addition of nickel (39-41%) and manganese (1-2%).
By the way, the markings of bronze alloys do not indicate the percentage of copper, it is calculated. For example, BrA9Zh3L contains aluminum – 9% and iron – 3%. The letters “Br” mean bronze, and “L” means foundry.
Appearance
Due to its high zinc content, brass is similar in color to real gold. However, the shade directly depends on the percentage of a particular chemical element. Therefore, the spectrum of colors can vary from pink-red to golden yellow. Typically, brass appears as a yellow-golden metal.
A bronze alloy has a silvery-white tone if it contains more than 35% tin. If its content reaches 40%, then the color of the metal is closer to white, reminiscent of steel. Products made from such bronze have a silver color with a light golden tint. If the composition contains a large proportion of copper (more than 85%), then the color of this metal is closer to red or dark brown.
Weight and strength
Brass is a more fragile and less durable material, prone to rapid wear. It is not used where high abrasion resistance is required. Due to the low density of zinc, brass is much lighter than bronze. Bronze is a wear-resistant and durable material. Due to its ductility, it is a favorite casting alloy for sculptors. It is much harder and stronger than brass. For example, a metal with 27% tin content is extremely hard, heavy and brittle. That is, the hardness of bronze depends on the percentage of tin in it. But this statement cannot apply to tin-free alloys.
More accurately, the specific gravity can be calculated by multiplying the density of the metal by the volume of the workpiece.
Properties
Brass needs to improve its qualities, so various additional components are introduced into the alloy. Thanks to this alloying, the cast brass alloy is characterized by resistance to corrosion, low coefficient of friction, increased fluidity, low tendency to segregation, and excellent technological and mechanical properties.
Bronze has high strength properties and a low coefficient of friction. Due to its excellent resistance to the negative effects of aggressive environments, the metal is widely used in shipbuilding and shipping. Copper alloy has a wide range of applications - from decorative interior elements to critical parts.
Price
Products made of brass are characterized by low cost. This is especially important to know when delivering scrap metal to raw material collection points. However, price is not the determining criterion, since it depends on the copper concentration. The more it is, the more expensive the metal. And for bronze, the determining factor is also the tin content. For example, tin bronze is valued higher than silicon bronze.
What is more expensive, brass or bronze?
The cost of metals on the market is almost the same. In some collection points the price of bronze may be higher than brass alloys, in others it’s the opposite. If you want to know the cost, go to the "Sell Scrap Brass" section. Considering the average market value, we can note:
- Increased demand for brass. This metal is more often used for the manufacture of household products, plumbing equipment, pipes and other common goods.
- Components. Brass alloys are mainly made using zinc, bronze alloys - with tin and a number of other additives.
- Characteristics. With properties and physico-chemical data it is more difficult. Here the parameters depend on the percentage of copper content and additional components.
Considering these factors, the demand and composition of brass metals is more in demand, therefore the average price of this metal is 10–20 rubles per kg higher than that of bronze scrap.
Comparison of two metals
As mentioned above, copper is used to make bronze and brass. However, its combination with tin or zinc produces alloys that have different properties and are used in different fields.
For example, bronze is considered a material that sculptors like to use to create busts, fences, monuments and other solutions that require durability and beauty. Brass is practically not used for such purposes; it is only occasionally used to create some kind of artistic products. The reason is the plasticity of the metal; it wears out quite quickly, while bronze monuments can stand for centuries.
An interesting fact is that bronze products have been used in maritime affairs since ancient times. They remarkably withstand the negative effects of salt water , while pure brass is completely incapable of this. To achieve certain properties, alloying with aluminum, lead or tin is required.
The appearance of these alloys is also slightly different. Bronze has a coarse-grained dark brown structure. Brass is much lighter; due to its characteristic yellowness, it resembles gold, and its structure is fine-grained.
In addition, both alloys are divided into different groups:
- brass can be two-component or multi-component;
- bronze - tin and tinless.
Which is better to choose?
If there is no way to identify the alloy, you can check the metal product in certified centers - and this will be the best choice of method for determining the composition. Such institutions have compact laboratories where spectral analysis is carried out using special equipment. This method allows you to determine the chemical composition of the material with maximum accuracy. To check, a special tool is used - a steeloscope. The method is characterized by high sensitivity and the ability to determine the composition without changing the structure of the metal.
Usually, metal reception points also have such equipment. It is used for fast and accurate identification of ferrous and non-ferrous alloys. This method is also good because even a small specimen is enough to use it.
To learn how to distinguish between metals, watch the following video.
Differences in the nature of the fracture and evaluation of the finished product
Many people will even say, why bother figuring out whether it’s brass or copper if the two alloys look almost the same? But the fact is that this is important for many, in particular for people who will be involved in making some kind of sculptures or melting them down. Accordingly, very often a distinction is required if you are going to sell metal for scrap.
The fact is that brass is cheaper than bronze, so at a metal collection point they can simply deceive you and offer you a smaller amount. If the weight is small, then the losses will be insignificant, but if you have a fairly large amount of goods, then you will lose a decent amount of money. It is worth noting that there is no need to conduct tests, just look at the finished products. Brass is almost never used in shipping.
Locksmith tool
This material is destroyed when exposed to sea salt water, so compasses and some parts in shipbuilding are used exclusively in bronze. Therefore, if they are trying to deceive you, insist on checking the product, or contact a certified center. They usually have collection points as well as small compact laboratories. They can conduct a quick, simple analysis and analyze the product using laboratory equipment.
It is quite easy to distinguish between metals when viewing the fracture site. Brass breaks in fairly small grains, bronze breaks off in large pieces and has a coarse grain. At the same time, the color of the bronze fracture has a reddish tint; if it is brass, then it has a whitish or yellowish tint.
Accessories skull
Unfortunately, these methods cannot be used at home due to the lack of laboratory equipment. Magnet and chip tests are available for home users. They are also very informative.
The difference between the two metals
The difference between these two alloys is as follows:
- Bronze is obtained by alloying copper with tin. In addition, this alloy contains impurities of metals such as lead, aluminum, beryllium, silicon, etc. Brass is obtained by fusing copper with zinc. Iron, nickel, manganese, lead, etc. are also added to this alloy.
- Bronze is a dark brown metal with a coarse grain structure, while brass is yellow in color and is quite smooth and fine grained.
- When interacting with sea water, bronze products do not deteriorate at all, but brass products may suffer. This property of alloys is taken into account in the construction of ships and the manufacture of various fishing accessories.
- Bronze is divided into tin and tin-free groups, and its opponent is divided into two-component and multi-component.
- Bronze products are much stronger than brass and they are much more resistant to wear.
- Bronze is often used to make fences, monuments and a variety of metal interior decorations. Brass is also used to make various jewelry and decorative elements, but quite rarely. But it is used to create steel-brass - a fairly practical bimetal that is not prone to rust.
Thus, it is not so easy to distinguish bronze from brass. It’s not easy to do this at home, but it’s possible. You just need to carefully examine both alloys , which are located in the same place. If you pick them up, bronze will be much heavier than brass, and its color will be much darker.
This is interesting: Brass profile: main types and scope of application
Determining the type of alloy by physical properties
Comparing brass and bronze, one can note a number of natural physical differences:
- Weight. Bronze alloys are heavier because they contain tin, which is heavier than zinc. This statement applies only to tin bronzes, and tin-free bronze alloys cannot be determined by this criterion.
- Bronze, when in contact with sea water, practically does not react to its influence, that is, it does not corrode. This cannot be said about brass, which must be alloyed to protect it from negative influences.
- Due to the presence of soft tin in the alloy, bronze has high anti-friction properties. This indicator can be called the main difference between bronze and brass.
General characteristics of metals
Brass and bronze are two similar-looking copper-based alloys from which many decorative and technical products are made. Both metals have a low melting point, which allows you to make different products from them with your own hands. Despite the similarities, they have completely different chemical composition, color and physical properties. However, it is quite difficult for an ordinary person not involved in metallurgy to identify them.
Brass
It is based on zinc, sometimes with the addition of other elements (nickel, tin, manganese, lead, iron, bismuth and others). Metal was known long before our era. Due to its color reminiscent of gold, brass was used to mint ancient Roman coins, various household items and jewelry. In the modern world, the alloy is most often used to produce steel-brass bimetal, from which art products and decorative fittings are made.
Brass is not resistant to abrasion, but is characterized by high ductility and good anti-corrosion properties. Easily lends itself to various types of welding (gas, arc) and is easily rolled. Products made from it have a yellowish color and are well polished. It is not ferromagnetic. Particularly popular is a type of wrought brass alloy called tombac. It contains 88-97% copper, and the rest is zinc. Due to its high plasticity, it is widely used in artistic casting, for the manufacture of insignia, and wind instruments.
This alloy is often used to imitate gold. Nowadays, school gold medals are made from it, coated with real gold.
Bronze
This is a copper alloy, where the main element is tin or other chemical elements (nickel, aluminum, silicon and the like). But high-quality bronze is obtained only in combination with tin. Metal appeared in human life at the beginning of the Bronze Age. The oldest products made from it date back to the 5th millennium BC. Its classic use in the recent past was the casting of bells and cannons.
In the molten state, the metal has good fluidity, which allows it to be cast into any shape, even the most complex . Due to its high resistance to mechanical abrasion and corrosion resistance, the material is used in mechanical engineering, rocketry, aviation, and shipbuilding. And due to the fact that the alloy is not exposed to the negative effects of atmospheric phenomena, it is used for casting sculptures, monuments, and decorative elements of the exterior.
Areas of application of copper alloys
It is reasonable to start analyzing the differences between the two alloys with a short excursion into history. Initially, copper was actively used for the manufacture of household utensils, various jewelry, weapons, household items, and coins. Later it was partially replaced by iron.
Currently, bronze alloys are not so actively used, but are still used in various fields:
- Interior elements, decorative items, lamp parts, stationery, mirror and picture frames, and miniature boxes are cast from bronze.
- Industrial production supplies gears, bearings, bushings, O-rings, various gaskets for automobiles and various communication systems.
- Bronze alloy with the addition of aluminum is in demand for the manufacture of parts for equipment operating in aggressive environments (chemical laboratories, water supply systems, units with increased operating pressure).
- Modern electronics require the use of bronze for fiber optic equipment, microcircuits, and small parts.
The material is in demand due to its durability, ductility, resistance to corrosion and aggressive external influences. Bronze is still used for the production of some plumbing products: fittings, taps, handles and support legs for bathtubs, wiring and assemblies in water pipes. Application is limited due to the high cost of this alloy.
Brass is used more actively in various sectors of the national economy. The alloy is well soldered to different grades of steel, has corrosion resistance, ductility and a large margin of safety.
Brass is used to produce parts for cars, river and sea vessels, water supply and heat supply systems, equipment for the chemical industry, and gas pipelines.
In household use, brass couplings, tees, fittings, nipples, faucets, decorative elements and even jewelry are often found.
Pure physics
The density of copper alloys is the next criterion for distinguishing brass from bronze. However, the popular belief that scales will give a definite answer is incorrect. Confirmation of this is provided by connection densities:
- rolled brass – 8.4 – 8.7;
- yellow brass – 8.43;
- bronze – 7.4 – 8.9.
All values are given in g/cc. As you can see, the weight of bronze, like its color, is highly dependent on the tin content. When its inclusion is at the level of 8%, the connection density is minimal and lower than that of brass. An increase in tin content leads to a heavier alloy. The result is that such bronze weighs more than brass. Therefore, using mass as a distinguishing criterion for copper alloys is not recommended in practice.
This video outlines the principle of calculating and determining metal based on weight and density:
The main differences between the alloys
Despite their similar appearance due to the use of copper as a base, bronze and brass have certain differences due to the addition of tin and zinc. Thanks to this, the scope of application of both materials is quite wide and varied.
Bronze is used quite often by sculptors. It is excellent for the production of monuments, sculptures, busts, fences and other artistic products. It can stand for hundreds of years without changing its shape or structure. Brass is used quite rarely for such purposes, which is due to the high ductility of this alloy, which negatively affects the durability and wear resistance of sculptures.
Brass and bronze
Due to its properties, one of which is resistance to salty sea water, bronze was previously widely used in maritime affairs. In order for brass to achieve the same properties, it is necessary to add alloying components such as aluminum, tin or lead.
Despite the external similarity, there are small differences between bronze and brass that can be seen with the naked eye. The main thing that should be highlighted is the difference between bronze and brass in color. Bronze has a dark brown tint, while brass is lighter, reminiscent of gold due to its yellowish tint.
The main differences between these two alloys should be highlighted:
- Bronze is produced by fusing copper and tin, with the possible addition of various impurities. Brass is obtained by producing an alloy of copper and zinc, but just like bronze, it can contain additional components.
- Bronze has a coarse-grained structure, brass, in turn, is fine-grained and quite smooth. You can see the structure by examining metal products at a fracture.
- Bronze has a dark brown tint, brass has a yellowish tint.
- Bronze is resistant to aggressive external environments, while brass can be destroyed even when exposed to sea water. This is the diversity of applications of alloys.
- Products made from bronze are much stronger and heavier than brass, and are also characterized by increased wear resistance.
- Due to its properties, bronze is used much more often in industry, but brass is used as part of the steel-brass bimetal, the properties of which exceed those of bronze.
Despite many differences, it is quite difficult to determine in everyday life what alloy a product is made of, but using several methods you can cope with this task.
Properties and color of bronze and brass with photos
Multicomponent alloys differ from each other in shades. If the first has a dark brown palette, then the second gives itself away as yellow, similar to a precious metal.
If we talk about characteristics, bronze raw materials have higher anti-corrosion, elasticity and strength. Salt water, air, carbon dioxide solutions and various salts are not able to change the appearance or deform the product. In addition, it is easy to weld and soft solder.
Depending on the use of alloying additives, the characteristics also change. For example, silicon and manganese significantly increase heat resistance, and lead, phosphorus and zinc increase anti-friction properties. The addition of iron and nickel leads to a decrease in grain structure and a decrease in electrical conductivity.
The disadvantages include:
- relatively low hardness;
- fragility;
- high specific gravity;
- high price (compared to aluminum).
If we talk about brass, its features are in many ways similar to bronze. Corrosion resistance is not sufficiently satisfactory, because as the temperature rises, the rate of destruction of the metal also increases. A variety of factors can provoke this. Impact strength decreases greatly with increasing processing temperature. Electrical and thermal conductivity indicators are significantly lower than those of a bronze alloy.
However, if tin is present in the composition, then the anti-corrosion properties and strength are such that parts for marine vessels are made from this material. And the most common are lead brass, since they have good cutting properties and can be processed on automatic machines. The advantages also include high fluidity during melting and a low degree of shrinkage during casting.
But there are also some disadvantages. When manufacturing parts using the injection molding method, large volume cavities may appear. During smelting, zinc has the ability to evaporate and react with oxygen. To prevent this, a layer of coal is poured into the crucible. Special fluxes must be added to the main composition. If you do not take effective measures, this will result in a loss of finances, since the waste will have to be recycled.
The main differences between bronze and brass: how to distinguish them
The basis of the two materials, as we wrote above, is copper. They differ in chemical composition and characteristics. But by appearance it is difficult to say exactly what kind of metal is in front of your eyes. Only a specialist can determine this by the fracture of the product. To summarize the above, we can find the differences between the two alloys:
- The main alloying element for bronze is tin, and for brass it is zinc. But both are made from Cu.
- The first material has the best anti-corrosion properties (it resists the effects of salt water, air, and aggressive environments well). Additional alloying elements can improve this quality.
Nuances of spectral comparative analysis
Due to the variety of copper-based alloys, it is difficult to accurately determine the type of their connection. Any of the methods to distinguish brass and bronze, even the most effective, does not provide a 100% guarantee. If you need an accurate answer to the question of what kind of alloy it is, then the only way to a reliable answer is to use spectral analysis. You can contact a scrap metal collection point, which may have the appropriate equipment.
Spectral analysis allows you to determine the chemical composition of a metal alloy based on its spectrum. In addition, this method has other advantages:
- high sensitivity;
- accuracy of results;
- studying the composition of brass and bronze products without destroying their structure;
- you can study the composition even on a small sample.
To carry out spectral analysis, a special tool is used - a steeloscope. It is designed for rapid visual qualitative and quantitative assessment of ferrous and non-ferrous alloys in the visible region of the spectrum.
Heat treatment
Temperature 600-650 °C is critical for zinc. The metal oxidizes when heated this way. This is a real way to visually distinguish bronze from brass in a burner flame:
- Bronze. The alloy will simply heat up. Its color and mechanical properties will remain unchanged. Attempting to bend a bronze specimen may result in its destruction.
- Brass. Oxidation of zinc causes an ashy coating on the surface of the joint. Additionally, after heat treatment at 600 °C, brass becomes ductile, and the alloy sample does not break when bent.
All that remains is to find a powerful burner. Here a gas stove or a lighter flame will not be enough.
Video - Melting bronze and brass:
Chemical technique
The use of reagents is an effective but destructive way to distinguish between copper alloys. Chemical analysis takes place in several stages:
- The shavings are removed from brass and bronze.
- An aqueous nitric acid solution is prepared with a 1:1 ratio.
- The shavings are placed in various containers filled with an acid reagent.
- Each tank is heated to a boil after the chips have completely dissolved.
- The compositions are kept in a boiling state over low heat for 30 minutes.
The result is that the container with brass remains transparent, while a white tin precipitate forms in the bronze container. Naturally, the technology is not suitable for tin-free alloys.
Chemical reaction to determine alloy type
The chemical method is the most reliable. During the work, the areas under study will be subject to destruction, and therefore you should not expose the entire subject of study to reagents; a small piece will be enough.
To carry out the analysis, you will need nitric acid, when working with which you must observe safety precautions and avoid getting the solution on open areas of the body, since the reagent is highly aggressive.
The procedure consists of several successive steps:
- The process continues until the metallic material is completely dissolved.
- A small piece of the test material is prepared.
- Mix nitric acid and water in equal proportions.
- A specimen of the alloy is placed in the resulting solution.
- The liquid is heated to boiling point.
How to distinguish at home?
In practice, there are several simple and proven methods to help identify metals.
Heating
Heat treatment helps distinguish copper alloys from each other. It is carried out using the flame of a powerful burner. To do this, the metal sample is heated to 600-650?C. A fire or the burner of a regular gas stove does not provide sufficient temperature. If, as a result of the procedure, an ash coating (zinc oxide) appears on the surface of the product, and the material itself becomes plastic, then it is brass.
When bending, metal does not break, but bends. Such plasticity and pliability is associated with the presence of zinc in it. If the product became hot when heated, but did not change either color or other mechanical characteristics, this indicates bronze. When bent, it tends to break.
Filing
The essence of the method is to determine the alloy by the quality of the chips formed by filing a metal product. A hacksaw is used as a tool. Brass is sawn in layers, forming shaped shavings. And bronze, due to its fragility, is sawn into small flakes, more reminiscent of dust.
Using a magnet
Not all copper alloys are ferromagnetic. For example, tin and lead tend to be attracted to a magnet, but it has no effect on brass. This testing method requires a strong magnet (for example, neodymium), which must be brought alternately to products made of different materials. Bronze will stick slightly due to the tin, iron or nickel it contains. The higher the content of these components in the metal, the more the bronze product tends to be magnetized. For example, a metal marked BrAZHN-10-4-4, where the digital designation indicates the content of Fe (4%) and Ni (4%), has maximum magnetic susceptibility.
Classic brass does not react in any way to neodymium. However, brass alloys containing iron and nickel (LAZh and LAN), respectively, will also be attracted to the magnet. All these facts call into question the effectiveness of the method itself.
Determination by the nature of the fracture
Sometimes, when it is not possible to determine the metal by other means, this can be done by visual inspection of the fracture site. Brass tends to break into small whitish or yellowish grains. The bronze alloy breaks off in large pieces with a coarse grain structure. The color on the cut has a characteristic reddish tint.
Chemical treatment
Another effective method to distinguish copper alloys from each other is by exposing them to a chemical reagent. All you need is the necessary special equipment and 50% nitric acid (HNO3). Test tubes containing a mixture of reagent and metal shavings are heated until a white tin precipitate appears, the presence of which indicates bronze. If the liquid remains transparent, this means that there is brass in it.
However, this method is not suitable for tin-free alloys. If nitric acid is not available, it can be replaced with a solution of sea salt. In this case, the brass shavings will change their color, but the bronze shavings will remain without visible changes.
Welding machine
For brass, the welding process will be accompanied by the formation of white smoke generated due to the burnout of zinc. With bronze, as a result of contact with the welding arc, no smoke will be observed.