What is thermal conductivity
This term refers to the ability of various materials to exchange energy, which in this case is represented by heat. In this case, energy transfer passes from the hotter part to the colder part and occurs due to:
- Molecules
- Atoms.
- Electrons and other particles of the metal structure.
The thermal conductivity of stainless steel will differ significantly from that of another metal - for example, the thermal conductivity of copper will be different than that of steel.
To indicate this indicator, a special value is used, called the thermal conductivity coefficient. It is characterized by the amount of heat that can pass through a material in a certain unit of time.
The concept of thermal conductivity
It is an intensive physical quantity, that is, a quantity that describes a property of matter that does not depend on the amount of the latter. Intensive quantities are also temperature, pressure, electrical conductivity, that is, these characteristics are the same at any point of the same substance. Another group of physical quantities are extensive, which are determined by the amount of matter, for example, mass, volume, energy and others.
The opposite value for thermal conductivity is thermal resistance, which reflects the ability of a material to prevent the transfer of heat passing through it. For an isotropic material, that is, a material whose properties are the same in all spatial directions, thermal conductivity is a scalar quantity and is defined as the ratio of the heat flux through a unit area per unit time to the temperature gradient. Thus, a thermal conductivity of one watt per meter Kelvin means that one Joule of thermal energy is transferred through the material:
- in one second;
- across an area of one meter square;
- at a distance of one meter;
- when the temperature difference on surfaces located one meter apart in a material is equal to one Kelvin.
It is clear that the higher the thermal conductivity value, the better the material conducts heat, and vice versa. For example, the value of this value for copper is 380 W/(m*K), and this metal transfers heat 10,000 times better than polyurethane, whose thermal conductivity is 0.035 W/(m*K).
Properties of copper Cu: thermal conductivity and density of copper
The table shows the thermophysical properties of copper depending on temperature in the range from 50 to 1600 degrees Kelvin.
The density of copper is 8933 kg/m3 (or 8.93 g/cm3) at room temperature. Copper is almost four times heavier than aluminum and iron. These metals will float on the surface of the liquid copper. The copper density values in the table are indicated in kg/m3.
The dependence of copper density on its temperature is presented in the table. It should be noted that the density of copper decreases when it is heated, both as a solid metal and as a liquid copper. The decrease in the density of this metal is due to its expansion when heated - the volume of copper increases. It should be noted that liquid copper has a density of about 8000 kg/m3 at temperatures up to 1300°C.
The thermal conductivity of copper is 401 W/(m deg) at room temperature, which is a fairly high value among metals, comparable to the thermal conductivity of silver.
At 1357K (1084°C) copper goes into a liquid state, which is reflected in the table by a sharp drop in the value of the thermal conductivity coefficient of copper. It can be seen that the thermal conductivity of liquid copper is almost two times lower than that of solid metal.
The thermal conductivity of copper tends to decrease when it is heated, but at temperatures above 1400 K, the thermal conductivity value begins to increase again.
The table discusses the following thermophysical properties of copper at various temperatures:
- copper density, kg/m3;
- specific heat capacity, J/(kg deg);
- thermal diffusivity, m2/s;
- thermal conductivity of copper, W/(m K);
- electrical resistivity, Ohm m;
- Lorentz function;
- heat capacity ratio.
Thermal conductivity of steel, copper, aluminum, nickel and their alloys
Ordinary iron and non-ferrous metals have different structures of molecules and atoms. This allows them to differ from each other not only in mechanical properties, but also in thermal conductivity properties, which, in turn, affects the use of certain metals in various sectors of the economy.
table 2
Steel has a thermal conductivity coefficient at an ambient temperature of 0 degrees. (C) equal to 63, and when the degree increases to 600, it decreases to 21 W/m*degree. Aluminum, under the same conditions, on the contrary, will increase the value from 202 to 422 W/m*deg. Aluminum alloys will also increase thermal conductivity as the temperature increases. Only the value of the coefficient will be an order of magnitude lower, depending on the amount of impurities, and range from 100 to 180 units.
Copper, with a temperature change within the same limits, will reduce thermal conductivity from 393 to 354 W/m*deg. At the same time, copper-containing brass alloys will have the same properties as aluminum ones, and the thermal conductivity value will vary from 100 to 200 units, depending on the amount of zinc and other impurities in the brass alloy.
The thermal conductivity coefficient of pure nickel is considered low; it will change its value from 67 to 57 W/m*deg. Alloys containing nickel will also have a coefficient with a reduced value, which, due to the content of iron and zinc, ranges from 20 to 50 W/m*deg. And the presence of chromium will reduce the thermal conductivity in metals to 12 units, with a slight increase in this value when heated.
Pros and cons of aluminum radiators
By comparing the strengths and weaknesses of devices, you can understand their main differences. After all, the difference between copper and aluminum radiators lies in their main characteristics. What is considered an objective advantage for one person turns out to be a serious disadvantage for another. Just look at the pros and cons of aluminum products and you will understand the difference between them.
Let's start with the positive aspects of aluminum as a material for the manufacture of car heater radiators.
- Price. While cost was considered a disadvantage of copper radiators, here it is a serious advantage. If we compare the price tags for both products, aluminum ones will win by about 2 times. Much depends on the manufacturer, but still the difference in cost remains significant. The buyer can save a lot. Because of this, aluminum units have such a large audience.
- Heat dissipation. Provided that the number of plates is increased, that is, the cooling area becomes larger, aluminum will not be much inferior to copper in terms of heat transfer. Therefore, in this component they are practically the same. But remember that aluminum ones are cheaper.
- Range. A huge proportion of modern cars that have been produced over the past few years are equipped with aluminum units from the factory. Because of this, the number of their analogues and original spare parts offered by different manufacturers is growing. The copper versions have a more modest choice.
We're done with the benefits. Let's move on to the other side of the coin. Aluminum is not doing so well. The stated advantages are beyond doubt. But still, motorists make a choice in favor of copper after they have studied the main disadvantages of the design option under consideration.
Therefore, the disadvantages should definitely be pointed out. This clearly shows the differences between the elements. The main disadvantages include:
- Thermal conductivity indicators. This is a very important drawback that literally negates all the objective positive qualities of the devices. If the driver needs to get the most efficient radiator so that the heating system works efficiently and fully warms up the interior, he will not look in the direction of aluminum.
- Repairability
Approximately the same conclusions can be drawn regarding these devices, which are made from two different materials.
Which conducts heat better: aluminum or copper?
Today, radiators are made from a variety of materials, the most common being steel, stainless steel and aluminum.
Always have doubts about which radiator to choose for installation in your home? Obviously, this depends on personal taste, as well as on the requirements that you have set for yourself regarding the quality of the heating of the room.
Aluminum is by far the most environmentally friendly material and has a huge number of advantages.
Differences between copper and aluminum
The main concerns regarding winding material selection reflect five characteristic differences between copper and aluminum:
Table: Five characteristic differences between copper and aluminum
| Parameter | Aluminum | Copper |
| Expansion coefficient per °C x 10 -6 at 20 °C | 23 | 16,6 |
| Thermal Conductivity BTU/ft/h/FFT 2/°F at 20°C | 126 | 222 |
| Electrical conductivity % at 20°C | 61 | 101 |
| Tensile strength n/mm 2 (soft) | 28-42 | 40 |
How to choose a heating radiator: expert advice
In this article we will not consider cast iron radiators, because... they are losing popularity among buyers.
Let's focus on the most popular models.
The material will tell you in detail about the advantages of aluminum and steel batteries.
Aluminum radiators are lightweight
Aluminum radiators are lighter than traditional steel or cast iron radiators, this fact makes it possible to place such a radiator on any wall in the room.
Aluminum batteries can be hung on the wall, even in situations where the thickness does not allow for deep fastening.
This significantly saves the cost of paying for construction work, since they can be hung very quickly and reliably.
Aluminum is a corrosion resistant material
Aluminum is not subject to corrosion, which makes it an ideal material for the production of radiators that are intended to be installed in areas such as bathrooms and kitchens where there is high humidity.
This is interesting: Equal-flange angle GOST 8509 93. Defining assortment of angle
Aluminum conducts heat well
Aluminum heats up quickly, making it an excellent heat conductor.
Aluminum radiators have a low water content, which means that once turned on, such devices give an intense burst of heat and heat up rooms quite quickly.
By installing aluminum radiators, you can quickly achieve the required temperature in the rooms, as they have the shortest response time.
The main advantage is a significant saving in energy costs during the heating season and, as a wonderful bonus, saving money, since aluminum radiators can be turned off while you are away from the house, and when you return home, turn them on and quickly get a warm home without spending a long time waiting.
Aluminum radiators come in a wide range of designs and colors
There is a common belief that efficient heat cannot be beautiful and original. Fortunately, the days when design must give way to superior performance are over.
Aluminum radiators have a diverse range of designs and offer even the most demanding buyer a decent choice.
You can choose your own finishing color that will perfectly match the style of your home, the shape of the radiator will be one hundred percent in harmony with your home or office atmosphere.
Stainless steel
The use of steel for the production of heat exchangers allows us to obtain durable products, which are mainly used for individual heating systems in houses and cottages.
Due to the ability to control the quality of the coolant and the pressure in the system, steel appliances will be an excellent choice for autonomous heating systems.
Provided that high-quality coolant is supplied and the working fluid pressure is moderate, such devices will last more than 30 years.
Connectivity
Oxides, chlorides, sulfides or base metals that are more conductive on copper than on aluminum. This fact makes cleaning and protecting aluminum connectors more important. Some consider copper and aluminum compounds to be incompatible. Also questionable is the mating of connections between the aluminum of the transformers and the copper connection wire.
Expansion coefficient
When temperature changes, aluminum expands almost a third more than copper. This expansion, along with the ductile nature of aluminum, causes some problems for improperly installed bolted connections.
To avoid loosening of the connection, it must be spring-loaded. By using either cup washers or pressure washers, the necessary elasticity during articulation can be achieved without compressing the aluminum.
When using proper fittings, aluminum connections can be equal in quality to copper ones.
Thermal conductivity
Some argue that since the thermal conductivity of copper is higher than aluminum, this has the effect of reducing the hot spot temperature of the transformer winding.
This is only true when the copper and aluminum winding conductors are of the same size, geometry and design.
Therefore, for any power transformer of a given size, the thermal conductivity characteristics of aluminum can be very similar to copper.
Characteristics of thermal conductivity of materials
The concept of thermal conductivity of materials is characterized by the ability to transfer thermal energy within a certain object from heated parts to cold ones. The process is carried out by atoms, molecules, electrons and occurs in any body with an uneven temperature distribution.
From the standpoint of kinetic physics, this process occurs as a result of the interaction of particles of molecules in hotter areas within the sample with other elements characterized by a lower temperature. The mechanism and rate of heat transfer depends on the state of aggregation of the substance.
The thermal conductivity category involves determining the heating rate of a material sample and the movement of a temperature wave in a certain direction. The indicator depends on physical parameters:
- density;
- temperature of phase transition to liquid state
- speed of sound propagation (for dielectrics).
Thermal conductivity of brass and bronze
The table shows the thermal conductivity values of brass, bronze, as well as copper-nickel alloys (constantan, copel, manganin, etc.) depending on temperature - in the range from 4 to 1273 K.
The thermal conductivity of brass, bronze and other copper-based alloys increases when heated. According to the table, L96 brass has the highest thermal conductivity of the alloys considered at room temperature. Its thermal conductivity at a temperature of 300 K (27°C) is 244 W/(m deg).
Also copper alloys with high thermal conductivity include: brass LS59-1, tombac L96 and L90, tin tombac LTO90-1, rolled tombac RT-90. In addition, the thermal conductivity of brass is generally higher than that of bronze. It should be noted that bronzes with high thermal conductivity include: phosphorus, chromium and beryllium bronzes, as well as BrA5 bronze.
The copper alloy with the lowest thermal conductivity is manganese bronze - its thermal conductivity coefficient at a temperature of 27°C is 9.6 W/(m deg).
The thermal conductivity of copper alloys is always lower than the thermal conductivity of pure copper, all other things being equal. In addition, the thermal conductivity of copper-nickel alloys is particularly low. The most thermally conductive of them at room temperature is cupronickel MNZhMts 30-0.8-1 with a thermal conductivity of 30 W/(m deg).
Table of thermal conductivity of brass, bronze and copper-nickel alloysAlloyTemperature, KThermal conductivity, W/(m deg)Copper-nickel alloysBrassBronze
| Beryllium copper | 300 | 111 |
| Constantan of foreign production | 4…10…20…40…80…300 | 0,8…3,5…8,8…13…18…23 |
| Constantan MNMts40-1.5 | 273…473…573…673 | 21…26…31…37 |
| Kopel MNMts43-0.5 | 473…1273 | 25…58 |
| Manganin of foreign production | 4…10…40…80…150…300 | 0,5…2…7…13…16…22 |
| Manganin MNMts 3-12 | 273…573 | 22…36 |
| Cupronickel MNZHMts 30-0.8-1 | 300 | 30 |
| Nickel silver | 300…400…500…600…700 | 23…31…39…45…49 |
| Automatic brass UNS C36000 | 300 | 115 |
| L62 | 300…600…900 | 110…160…200 |
| L68 deformed brass | 80…150…300…900 | 71…84…110…120 |
| L80 semi-tompak | 300…600…900 | 110…120…140 |
| L90 | 273…373…473…573…673…773…873 | 114…126…142…157…175…188…203 |
| L96 tombak drawn | 300…400…500…600…700…800 | 244…245…246…250…255…260 |
| LAN59-3-2 aluminum-nickel brass | 300…600…900 | 84…120…150 |
| LMC58-2 manganese brass | 300…600…900 | 70…100…120 |
| LO62-1 tin | 300 | 99 |
| LO70-1 tin | 300…600 | 92…140 |
| LS59-1 annealed brass | 4…10…20…40…80…300 | 3,4…10…19…34…54…120 |
| LS59-1V leaded brass | 300…600…900 | 110…140…180 |
| LTO90-1 tombak tin | 300…400…500…600…700…800…900 | 124…141…157…174…194…209…222 |
| BrA5 | 300…400…500…600…700…800…900 | 105…114…124…133…141…148…153 |
| BrA7 | 300…400…500…600…700…800…900 | 97…105…114…122…129…135…141 |
| BrAZhMC10-3-1.5 | 300…600…800 | 59…77…84 |
| BrAZHN10-4-4 | 300…400…500 | 75…87…97 |
| BrAZHN11-6-6 | 300…400…500…600…700…800 | 64…71…77…82…87…94 |
| BrB2, annealed at 573K | 4…10…20…40…80 | 2,3…5…11…21…37 |
| BrKd | 293 | 340 |
| BrKMTs3-1 | 300…400…500…600…700 | 42…50…55…54…54 |
| BrMC-5 | 300…400…500…600…700 | 94…103…112…122…127 |
| BrMTsS8-20 | 300…400…500…600…700…800…900 | 32…37…43…46…49…51…53 |
| BrO10 | 300…400…500 | 48…52…56 |
| BrOS10-10 | 300…400…600…800 | 45…51…61…67 |
| BrOS5-25 | 300…400…500…600…700…800…900 | 58…64…71…77…80…83…85 |
| BrOF10-1 | 300…400…500…600…700…800…900 | 34…38…43…46…49…51…52 |
| BrOTs10-2 | 300…400…500…600…700…800…900 | 55…56…63…68…72…75…77 |
| BrOTs4-3 | 300…400…500…600…700…800…900 | 84…93…101…108…114…120…124 |
| BrOTs6-6-3 | 300…400…500…600…700…800…900 | 64…71…77…82…87…91…93 |
| BrOTs8-4 | 300…400…500…600…700…800…900 | 68…77…83…88…93…96…100 |
| Aluminum bronze | 300 | 56 |
| Aged beryllium bronze | 20…80…150…300 | 18…65…110…170 |
| Manganese bronze | 300 | 9,6 |
| Production leaded bronze | 300 | 26 |
| Phosphor bronze 10% | 300 | 50 |
| Phosphor bronze annealed | 20…80…150…300 | 6…20…77…190 |
| Chromium bronze UNS C18200 | 300 | 171 |
Metal characteristics
The melting temperature of brass, depending on its composition, ranges from 880-950°C. Thus, with an increase in zinc impurity in the material under consideration, the melting point will decrease. It is worth noting that brass, due to its properties, can be welded well.
Brass is processed by resistance welding and can be rolled. The uncoated surfaces of the metal in question turn black when in contact with air. Brass has a yellow color and is highly polished. The non-ferrous metal in question can be melted within certain temperature limits, depending on the impurities in the composition of the material.
Metal Specifications
- Melting point – 880-950°C;
- Material density – 8,300-8,700 kg/cubic meter;
- Specific heat capacity - 0.377 kJ kg−1 K−1 at 20°C;
- Electrical resistivity - (0.07-0.08)·10−6 Ohm·m.
It is useful to know that bismuth, as well as lead, have a detrimental effect on brass, since they reduce the ability to deform when hot.
What are the advantages of non-ferrous metal, grade and application?
Brass belongs to the category of non-ferrous metals. It is useful to know about the chemical and physical benefits that brass has.
Advantages
- Corrosion resistance;
- High degree of fluidity;
- Excellent anti-friction properties;
- Slight tendency to segregation;
- Excellent technological properties;
- Excellent mechanical properties.
This is interesting: Steel nitriding: process technology, equipment
The list presented above does not limit the advantages and beneficial properties of this metal. You should not ignore the most popular brands of material, as well as application.
Melting point of brass
The melting point of brass of the considered brands varies in the range from 865 to 1055 °C. The most fusible is manganese brass LMts58-2 with a melting point of 865°C. Low-melting brasses also include: L59, L62, LAN59-3-2, LKS65-1.5-3 and others.
L96 brass has the highest melting point (1055°C). Among the refractory brasses, according to the table, we can also distinguish: brass L90, LA85-0.5, tin tombac LTO90-1.
Melting point of brassBrass, °СBrass, °С
| L59 | 885 | LMts55-3-1 | 930 |
| L62 | 898 | LMts58-2 manganese brass | 865 |
| L63 | 900 | LMtsA57-3-1 | 920 |
| L66 | 905 | LMtsZh52-4-1 | 940 |
| L68 deformed brass | 909 | LMtsOS58-2-2-2 | 900 |
| L70 | 915 | LMtsS58-2-2 | 900 |
| L75 | 980 | LN56-3 | 890 |
| L80 semi-tompak | 965 | LN65-5 | 960 |
| L85 | 990 | LO59-1 | 885 |
| L90 | 1025 | LO60-1 | 885 |
| L96 tombak drawn | 1055 | LO62-1 tin | 885 |
| LA67-2.5 | 995 | LO65-1-2 | 920 |
| LA77-2 | 930 | LO70-1 tin | 890 |
| LA85-0.5 | 1020 | LO74-3 | 885 |
| LAZ60-1-1 | 904 | LO90-1 | 995 |
| LAZHMts66-6-3-2 | 899 | LS59-1 | 900 |
| LAN59-3-2 aluminum-nickel brass | 892 | LS59-1V leaded brass | 900 |
| LANKMts75-2-2.5-0.5-0.5 | 940 | LS60-1 | 900 |
| LZhMts59-1-1 | 885 | LS63-3 | 885 |
| LK80-3 | 900 | LS64-2 | 910 |
| LKS65-1.5-3 | 870 | LS74-3 | 965 |
| LKS80-3-3 | 900 | LTO90-1 tombak tin | 1015 |
Melting point of bronze
The melting point of bronze ranges from 854 to 1135°C. Bronze AZHN11-6-6 has the highest melting point - it melts at a temperature of 1408 K (1135°C). The melting point of this bronze is even higher than the melting point of copper, which is 1084.6°C.
Bronzes with a low melting point include: BrOTs8-4, BrB2, BrMTsS8-20, BrSN60-2.5 and the like.
Melting temperature of bronze Bronzet, °С Bronzet, °С
| BrA5 | 1056 | BrOS8-12 | 940 |
| BrA7 | 1040 | BrOSN10-2-3 | 1000 |
| BrA10 | 1040 | BrOF10-1 | 934 |
| BrAZH9-4 | 1040 | BrOF4-0.25 | 1060 |
| BrAZhMC10-3-1.5 | 1045 | BrOTs10-2 | 1015 |
| BrAZHN10-4-4 | 1084 | BrOTs4-3 | 1045 |
| BrAZHN11-6-6 | 1135 | BrOTs6-6-3 | 967 |
| BrAZhS7-1.5-1.5 | 1020 | BrOTs8-4 | 854 |
| BrAMTS9-2 | 1060 | BrOTsS3.5-6-5 | 980 |
| BrB2 | 864 | BrOTsS4-4-17 | 920 |
| BrB2.5 | 930 | BrOTsS4-4-2.5 | 887 |
| BrKMTs3-1 | 970 | BrOTsS5-5-5 | 955 |
| BrKN1-3 | 1050 | BrOTsS8-4-3 | 1015 |
| BrKS3-4 | 1020 | BrOTsS3-12-5 | 1000 |
| BrKTs4-4 | 1000 | BrOTsSN3-7-5-1 | 990 |
| BrMG0.3 | 1076 | BrS30 | 975 |
| BrMC5 | 1007 | BrSN60-2.5 | 885 |
| BrMTsS8-20 | 885 | BrSUN7-2 | 950 |
| BrO10 | 1020 | BrХ0.5 | 1073 |
| BrOS10-10 | 925 | BrTsr0.4 | 965 |
| BrOS10-5 | 980 | Cadmium | 1040 |
| BrOS12-7 | 930 | Silver | 1082 |
| BrOS5-25 | 899 | HOT alloy | 1075 |
Note: The melting and boiling points of other common metals are given in this table.
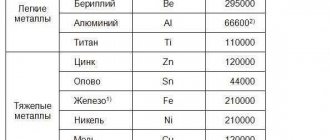
Thermal conductivity of non-ferrous metals and technical alloys
The table shows the thermal conductivity values of metals (non-ferrous), as well as the chemical composition of metals and technical alloys in the temperature range from 0 to 600°C.
Non-ferrous metals and alloys: nickel Ni, monel, nichrome; nickel alloys (according to GOST 492-58): cupronickel NM81, NM70, constantan NMMts 58.5-1.54, copel NM 56.5, monel NMZhMts and K-monel, alumel, chromel, manganin NMMts 85-12, invar; magnesium alloys (according to GOST 2856-68), electron, platinum-rhodium; soft solders (according to GOST 1499-70): pure tin, lead, POS-90, POS-40, POS-30, Rose alloy, Wood alloy.
The table shows that magnesium alloys and nickel have high thermal conductivity (at room temperature). Low thermal conductivity is characteristic of nichrome, invar and Wood's alloy.
Thermal conductivity coefficients of alloys
The table shows the thermal conductivity values of alloys in the temperature range from 20 to 200ºС. Alloys: aluminum bronze, bronze, phosphor bronze, invar, constantan, manganin, magnesium alloys, copper alloys, Rose alloy, Wood's alloy, nickel alloys, nickel silver, platinum-iridium, electron alloy, platinum-rhodium.
Thermal conductivity coefficient of other materials
MaterialHumidity mass fraction % W/(m•K)
| Bakelite varnish | — | 0,29 |
| Concrete with crushed stone | 8 | 1,28 |
| Plain paper | Air dry | 0,14 |
| Viniplast | — | 0,13 |
| Gravel | Air dry | 0,36 |
| Granite | — | 3,14 |
| Clay | 15-20 | 0,7-0,93 |
| Oak (along the grain) | 6-8 | 0,35-0,43 |
| Oak (across the grain) | 6-8 | 0,2-0,21 |
| Reinforced concrete | 8 | 1,55 |
| Cardboard | Air dry | 0,14-0,35 |
| Brickwork | Air dry | 0,67-0,87 |
| Leather | >> | 0,14-0,16 |
| Ice | — | 2,21 |
| Cork boards | 0,042-0,054 | |
| Freshly fallen snow | — | 0,105 |
| Snow compacted | — | 0,35 |
| The snow has begun to melt | — | 0,64 |
| Pine (along the grain) | 8 | 0,35-0,41 |
| Pine (across the grain) | 8 | 0,14-0,16 |
| Glass (ordinary) | — | 0,74 |
| Ftoroplast-3 | — | 0,058 |
| Ftoroplast-4 | — | 0,233 |
| Cinder concrete | 13 | 0,698 |
| Plaster | 6-8 | 0,791 |
Thermal conductivity coefficient of asbestos and foam concrete at different temperatures
(ρa=576kg/m3, ρп=400kg/m3,λ, W/(m•K))
Material-18oС0oС50oС100oС150oС
| Asbestos | — | 0,15 | 0,18 | 0,195 | 0,20 |
| Foam concrete | 0,1 | 0,11 | 0,11 | 0,13 | 0,17 |
Thermal conductivity coefficient of liquid W/(m•K) at different temperatures
Material0oС50oС100oС
| Aniline | 0,19 | 0,177 | 0,167 |
| Acetone | 0,17 | 0,16 | 0,15 |
| Benzene | — | 0,138 | 0,126 |
| Water | 0,551 | 0,648 | 0,683 |
| Vaseline oil | 0,126 | 0,122 | 0,119 |
| Castor oil | 0,184 | 0,177 | 0,172 |
| Methyl alcohol | 0,214 | 0,207 | — |
| Ethanol | 0,188 | 0,177 | — |
| Toluene | 0,142 | 0,129 | 0,119 |
Specific resistance and temperature coefficient of expansion (CTE) of metal wire (at 18ºС)
The table shows the values of electrical resistivity and CTE of metal wire made of various metals and alloys. Wire material: aluminum, tungsten, iron, gold, brass, manganin, copper, nickel, constantan, nichrome, tin, platinum, lead, silver, zinc. As can be seen from the table, nichrome wire has a high electrical resistivity and is successfully used as incandescent heating coils for many household and industrial devices.
Specific heat capacity of multicomponent special alloys
The specific (mass) heat capacity of multicomponent special alloys is given in the table at temperatures from 0 to 1300ºС.
Heat capacity dimension cal/(g deg). Heat capacity of special alloys: alumel, bell metal, Wood's alloy, Invar, Lipowitz alloy, Manganin, Monel, Rose alloy, phosphorus bronze, chromel, Na-K alloy, Pb - Bi alloy, Pb - Bi - Sn, Zn - Sn - Ni - Fe - Mn.
Table of thermal conductivity of thermal insulation materials
To make it easier to keep your house warm in winter and cool in summer, the thermal conductivity of walls, floors and roofs must be at least a certain figure, which is calculated for each region. The composition of the “pie” of walls, floor and ceiling, the thickness of the materials are taken into account so that the total figure is no less (or better yet, at least a little more) recommended for your region.
Heat transfer coefficient of modern building materials for enclosing structures
When choosing materials, it is necessary to take into account that some of them (not all) conduct heat much better in conditions of high humidity. If such a situation may occur for a long period of time during operation, the thermal conductivity for this condition is used in the calculations. The thermal conductivity coefficients of the main materials used for insulation are given in the table.
| Name of material | Thermal conductivity coefficient W/(m °C) | ||
| Dry | At normal humidity | At high humidity | |
| Woolen felt | 0,036-0,041 | 0,038-0,044 | 0,044-0,050 |
| Stone mineral wool 25-50 kg/m3 | 0,036 | 0,042 | 0,,045 |
| Stone mineral wool 40-60 kg/m3 | 0,035 | 0,041 | 0,044 |
| Stone mineral wool 80-125 kg/m3 | 0,036 | 0,042 | 0,045 |
| Stone mineral wool 140-175 kg/m3 | 0,037 | 0,043 | 0,0456 |
| Stone mineral wool 180 kg/m3 | 0,038 | 0,045 | 0,048 |
| Glass wool 15 kg/m3 | 0,046 | 0,049 | 0,055 |
| Glass wool 17 kg/m3 | 0,044 | 0,047 | 0,053 |
| Glass wool 20 kg/m3 | 0,04 | 0,043 | 0,048 |
| Glass wool 30 kg/m3 | 0,04 | 0,042 | 0,046 |
| Glass wool 35 kg/m3 | 0,039 | 0,041 | 0,046 |
| Glass wool 45 kg/m3 | 0,039 | 0,041 | 0,045 |
| Glass wool 60 kg/m3 | 0,038 | 0,040 | 0,045 |
| Glass wool 75 kg/m3 | 0,04 | 0,042 | 0,047 |
| Glass wool 85 kg/m3 | 0,044 | 0,046 | 0,050 |
| Expanded polystyrene (foam plastic, EPS) | 0,036-0,041 | 0,038-0,044 | 0,044-0,050 |
| Extruded polystyrene foam (EPS, XPS) | 0,029 | 0,030 | 0,031 |
| Foam concrete, aerated concrete with cement mortar, 600 kg/m3 | 0,14 | 0,22 | 0,26 |
| Foam concrete, aerated concrete with cement mortar, 400 kg/m3 | 0,11 | 0,14 | 0,15 |
| Foam concrete, aerated concrete with lime mortar, 600 kg/m3 | 0,15 | 0,28 | 0,34 |
| Foam concrete, aerated concrete with lime mortar, 400 kg/m3 | 0,13 | 0,22 | 0,28 |
| Foam glass, crumbs, 100 – 150 kg/m3 | 0,043-0,06 | ||
| Foam glass, crumbs, 151 – 200 kg/m3 | 0,06-0,063 | ||
| Foam glass, crumbs, 201 – 250 kg/m3 | 0,066-0,073 | ||
| Foam glass, crumbs, 251 – 400 kg/m3 | 0,085-0,1 | ||
| Foam block 100 – 120 kg/m3 | 0,043-0,045 | ||
| Foam block 121-170 kg/m3 | 0,05-0,062 | ||
| Foam block 171 – 220 kg/m3 | 0,057-0,063 | ||
| Foam block 221 – 270 kg/m3 | 0,073 | ||
| Ecowool | 0,037-0,042 | ||
| Polyurethane foam (PPU) 40 kg/m3 | 0,029 | 0,031 | 0,05 |
| Polyurethane foam (PPU) 60 kg/m3 | 0,035 | 0,036 | 0,041 |
| Polyurethane foam (PPU) 80 kg/m3 | 0,041 | 0,042 | 0,04 |
| Cross-linked polyethylene foam | 0,031-0,038 | ||
| Vacuum | 0 | ||
| Air +27°C. 1 atm | 0,026 | ||
| Xenon | 0,0057 | ||
| Argon | 0,0177 | ||
| Airgel (Aspen aerogels) | 0,014-0,021 | ||
| Slag | 0,05 | ||
| Vermiculite | 0,064-0,074 | ||
| Foam rubber | 0,033 | ||
| Cork sheets 220 kg/m3 | 0,035 | ||
| Cork sheets 260 kg/m3 | 0,05 | ||
| Basalt mats, canvases | 0,03-0,04 | ||
| Tow | 0,05 | ||
| Perlite, 200 kg/m3 | 0,05 | ||
| Expanded perlite, 100 kg/m3 | 0,06 | ||
| Linen insulating boards, 250 kg/m3 | 0,054 | ||
| Polystyrene concrete, 150-500 kg/m3 | 0,052-0,145 | ||
| Granulated cork, 45 kg/m3 | 0,038 | ||
| Mineral cork on a bitumen basis, 270-350 kg/m3 | 0,076-0,096 | ||
| Cork flooring, 540 kg/m3 | 0,078 | ||
| Technical cork, 50 kg/m3 | 0,037 | ||
Some of the information is taken from standards that prescribe the characteristics of certain materials (SNiP 23-02-2003, SP 50.13330.2012, SNiP II-3-79* (Appendix 2)). Those materials that are not specified in the standards are found on the manufacturers' websites. Since there are no standards, they can differ significantly from different manufacturers, so when purchasing, pay attention to the characteristics of each material you purchase.
The concept of thermal resistance and thermal conductivity coefficient
If thermal conductivity characterizes the ability of metals to transfer the temperature of bodies from one surface to another, then thermal resistance shows an inverse relationship, i.e. the ability of metals to prevent such transfer, in other words, to resist. Air has high thermal resistance. It is he who, most of all, prevents the transfer of heat between bodies.
The quantitative characteristic of the change in temperature of a unit area per unit of time by one degree (K) is called the thermal conductivity coefficient. The international system of units usually measures this parameter in W/m*deg. This characteristic is very important when choosing metal products that must transfer heat from one body to another.
| Metal | Thermal conductivity coefficient of metals at temperature, °C | ||||
| — 100 | 100 | 300 | 700 | ||
| Aluminum | 2,45 | 2,38 | 2,30 | 2,26 | 0,9 |
| Beryllium | 4,1 | 2,3 | 1,7 | 1,25 | 0,9 |
| Vanadium | — | — | 0,31 | 0,34 | — |
| Bismuth | 0,11 | 0,08 | 0,07 | 0,11 | 0,15 |
| Tungsten | 2,05 | 1,90 | 1,65 | 1,45 | 1,2 |
| Hafnium | — | — | 0,22 | 0,21 | — |
| Iron | 0,94 | 0,76 | 0,69 | 0,55 | 0,34 |
| Gold | 3,3 | 3,1 | 3,1 | — | — |
| Indium | — | 0,25 | — | — | — |
| Iridium | 1,51 | 1,48 | 1,43 | — | — |
| Cadmium | 0,96 | 0,92 | 0,90 | 0,95 | 0,44 (400°) |
| Potassium | — | 0,99 | — | 0,42 | 0,34 |
| Calcium | — | 0,98 | — | — | — |
| Cobalt | — | 0,69 | — | — | — |
| Lithium | — | 0,71 | 0,73 | — | — |
| Magnesium | 1,6 | 1,5 | 1,5 | 1,45 | — |
| Copper | 4,05 | 3,85 | 3,82 | 3,76 | 3,50 |
| Molybdenum | 1,4 | 1,43 | — | — | 1,04 (1000°) |
| Sodium | 1,35 | 1,35 | 0,85 | 0,76 | 0,60 |
| Nickel | 0,97 | 0,91 | 0,83 | 0,64 | 0,66 |
| Niobium | 0,49 | 0,49 | 0,51 | 0,56 | — |
| Tin | 0,74 | 0,64 | 0,60 | 0,33 | — |
| Palladium | 0,69 | 0,67 | 0,74 | — | — |
| Platinum | 0,68 | 0,69 | 0,72 | 0,76 | 0,84 |
| Rhenium | — | 0,71 | — | — | — |
| Rhodium | 1,54 | 1,52 | 1,47 | — | — |
| Mercury | 0,33 | 0,09 | 0.1 | 0,115 | — |
| Lead | 0,37 | 0,35 | 0,335 | 0,315 | 0,19 |
| Silver | 4,22 | 4,18 | 4,17 | 3,62 | — |
| Antimony | 0,23 | 0,18 | 0,17 | 0,17 | 0,21 |
| Thallium | 0,41 | 0,43 | 0,49 | 0,25 (400 0) | |
| Tantalum | 0,54 | 0,54 | — | — | — |
| Titanium | — | — | 0,16 | 0,15 | — |
| Thorium | — | 0,41 | 0,39 | 0,40 | 0,45 |
| Uranus | — | 0,24 | 0,26 | 0,31 | 0,40 |
| Chromium | — | 0,86 | 0,85 | 0,80 | 0,63 |
| Zinc | 1,14 | 1,13 | 1,09 | 1,00 | 0,56 |
| Zirconium | — | 0,21 | 0,20 | 0,19 | — |
This is interesting: What is scraping? Features and where is it used?
What does thermal conductivity depend on?
Studying the ability of heat transfer by metal products, it was revealed that thermal conductivity depends on:
- type of metal;
- chemical composition;
- porosity;
- sizes.
Metals have different crystal lattice structures, and this can change the thermal conductivity of the material. For example, in steel and aluminum, the structural features of microparticles affect differently the rate of transfer of thermal energy through them.
The thermal conductivity coefficient can have different values for the same metal when the exposure temperature changes. This is due to the fact that different metals have different melting degrees, which means that under other environmental parameters, the properties of the materials will also differ, and this will affect thermal conductivity.
Disadvantages of the high thermal conductivity of copper and its alloys
Copper has a much higher cost than brass or aluminum. At the same time, this metal has its disadvantages, which are directly related to its advantages. High thermal conductivity leads to the need to create special conditions during cutting, welding and soldering of copper elements. Since copper elements need to be heated much more concentrated compared to steel. Also, preliminary and concomitant heating of the part is often required.
Don’t forget that copper pipes require careful insulation if they make up the main line or distribution of the heating system. Which leads to an increase in the cost of network installation compared to options when other materials are used.
Example of thermal insulation of copper pipes
Difficulties also arise with gas welding of copper: this process will require more powerful torches. When welding metal 8–10 mm thick, two or three torches will be required. While one torch is used for welding, the other is heating the part. In general, welding work with copper requires increased costs for consumables.
It should also be said about the need to use special tools. So, to cut brass and bronze up to 15 cm thick, you will need a cutter capable of working with high-chromium steel 30 cm thick. Moreover, the same tool is enough to work with pure copper only 5 cm thick.
Factors influencing physical quantity
The ability to conduct heat depends on a number of factors, including the temperature, structure and electrical properties of the substance.
Material temperature
The effect of temperature on the ability to conduct heat differs for metals and nonmetals. In metals, conductivity is mainly due to free electrons. According to the Wiedemann-Franz law, the thermal conductivity of a metal is proportional to the product of the absolute temperature, expressed in Kelvin, and its electrical conductivity. In pure metals, electrical conductivity decreases with increasing temperature, so thermal conductivity remains approximately constant. In the case of alloys, electrical conductivity changes little with increasing temperature, so the thermal conductivity of alloys increases in proportion to temperature.
On the other hand, heat transfer in nonmetals is mainly associated with lattice vibrations and the exchange of lattice phonons. With the exception of high-quality crystals and low temperatures, the path of phonons in the lattice does not decrease significantly at high temperatures, and therefore the thermal conductivity remains constant over the entire temperature range, that is, it is insignificant. At temperatures below the Debye temperature, the ability of nonmetals to conduct heat, along with their heat capacity, decreases significantly.
Phase transitions and structure
When a material undergoes a first-order phase transition, for example from a solid to a liquid or from a liquid to a gas, its thermal conductivity may change. A striking example of such a change is the difference between this physical quantity for ice (2.18 W/(m*K) and water (0.90 W/(m*K).
Changes in the crystal structure of materials also affect thermal conductivity, which is explained by the anisotropic properties of various allotropic modifications of a substance of the same composition. Anisotropy affects different scattering intensities of lattice phonons, the main heat carriers in nonmetals, and in different directions in the crystal. A striking example here is sapphire, whose conductivity varies from 32 to 35 W/(m*K) depending on the direction.
Electrical conductivity
Thermal conductivity in metals changes along with electrical conductivity according to the Wiedemann-Franz law. This is due to the fact that valence electrons, moving freely throughout the crystal lattice of the metal, transfer not only electrical, but also thermal energy. For other materials, the correlation between these types of conductivity is not pronounced, due to the insignificant contribution of the electronic component to thermal conductivity (in nonmetals, lattice phonons play the main role in the mechanism of heat transfer).
Convection process
Air and other gases are, as a rule, good heat insulators in the absence of convection. This principle is the basis for the operation of many heat-insulating materials containing a large number of small voids and pores. This structure does not allow convection to spread over long distances. Examples of such man-made materials are polystyrene and silicide airgel. In nature, heat insulators such as animal skin and bird plumage work on the same principle.
Light gases such as hydrogen and gel have high thermal conductivities, while heavy gases such as argon, xenon and radon are poor conductors of heat. For example, argon, an inert gas that is heavier than air, is often used as an insulating gas filler in double-glazed windows and light bulbs. An exception is sulfur hexafluoride (SF6 gas), which is a heavy gas and has a relatively high thermal conductivity due to its high heat capacity.
Measurement methods
To measure the thermal conductivity of metals, two methods are used: stationary and non-stationary. The first is characterized by the achievement of a constant value of the changed temperature on the controlled surface, and the second - by a partial change in it.
Stationary measurement is carried out experimentally, requires a lot of time, as well as the use of the metal under study in the form of blanks of the correct shape, with flat surfaces. The sample is placed between the heated and cooled surface, and after touching the planes, the time during which the workpiece can increase the temperature of the cool support by one degree Kelvin is measured. When calculating thermal conductivity, the dimensions of the sample being studied must be taken into account.
Non-stationary research methods are used in rare cases due to the fact that the result is often biased. Nowadays, no one except scientists is involved in measuring the coefficient; everyone uses long-established experimental values for various materials. This is due to the constancy of this parameter while maintaining the chemical composition of the product.
Is it possible to increase the thermal conductivity of copper?
Copper is widely used in the creation of microcircuits for electronic devices and is designed to remove heat from parts heated by electric current. When trying to increase the speed of modern computers, developers were faced with the problem of cooling processors and other parts. One of the solutions was to split the processor into several cores. However, this method of combating overheating has exhausted itself, and now it is necessary to look for new conductors with higher thermal and electrical conductivity.
One solution to this problem is the recently discovered element graphene. Thanks to graphene deposition, the thermal conductivity of the copper element increases by 25%. However, the invention is still at the development level.
Importance in everyday life and production
Why is it important to consider thermal conductivity? A similar value is indicated in various tables for each metal and is taken into account in the following cases:
- In the manufacture of various heat exchangers. Heat is one of the important carriers of energy. It is used to provide comfortable living conditions in residential and other premises. When creating heating radiators and boilers, it is important to ensure rapid and complete heat transfer from the coolant to the end consumer.
- In the manufacture of outlet elements. You can often encounter a situation where you need to remove heat rather than supply it. An example is the case of heat removal from the cutting edge of a tool or gear teeth. To ensure that the metal does not lose its basic performance qualities, rapid removal of thermal energy is ensured.
- When creating insulating layers. In some cases, the material should not conduct thermal energy transfer. For such operating conditions, a metal is selected that has a low heat conductivity coefficient.
The indicator under consideration is determined when testing under various conditions. As previously noted, the thermal conductivity coefficient may depend on the operating temperature. Therefore, the tables indicate several of its values.
Heat transfer at the molecular level
When matter heats up, the average kinetic energy of its constituent particles increases, that is, the level of disorder increases, atoms and molecules begin to oscillate more intensely and with greater amplitude around their equilibrium positions in the material. Heat transfer, which at the macroscopic level can be described by Fourier's law, at the molecular level is the exchange of kinetic energy between particles (atoms and molecules) of a substance, without transfer of the latter.
This explanation of the mechanism of thermal conduction at the molecular level distinguishes it from the mechanism of thermal convection, in which heat transfer occurs due to the transfer of matter. All solids have the ability to conduct heat, while thermal convection is possible only in liquids and gases. Indeed, solids transfer heat mainly due to thermal conductivity, and liquids and gases, if there are temperature gradients in them, transfer heat mainly due to convection processes.