A metal product can be given shine in various ways. To do this, it is not necessary to use special coatings; you can use the polishing method. It can be mechanical, for example, using sanding wheels, chemical - when the metal is immersed in a special solution, and also electrochemical. In this case, the effects of chemical components and electrical discharges are combined, which trigger certain reactions or enhance them. Electrochemical polishing of metals can be performed in normal home conditions if you collect all the necessary equipment.
Process description
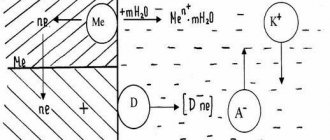
During electrochemical polishing, the treated metal surface acquires a mirror shine. Existing roughness is also reduced. The process goes like this:
- The part is considered an anode, that is, an electrode carrying a positive charge. It must be placed in a bath with a special composition.
- Another important component is the cathodes, which are necessary for the reaction to occur.
- As a result of exposure, a reaction occurs and dissolution occurs. It is uneven; first, the most noticeable roughness, which protrudes above the surface the most, is removed. At the same time, polishing occurs - the product acquires a mirror shine.
Removing noticeable large irregularities is called macropolishing, and smoothing out small defects is called micropolishing. If these processes occur simultaneously and evenly during processing, the product acquires shine and smoothness. It is also possible that shine will be obtained without smoothing or vice versa. The two types of polishing are not necessarily related.
Chemical polishing of metal results in the formation of a special film on the surface of the workpiece during the process. Its composition can be oxide or hydroxide. If it evenly covers the entire surface, this creates conditions for micropolishing. In this case, the outer part of the coating, located on the surface, continuously dissolves. To be able to carry out micropolishing, it is necessary to ensure that a balance is maintained between continuous coating formation and dissolution; while working with the part, the layer thickness must remain unchanged. This will allow the electrons of the metal being processed and the composition used to interact in the process without the danger of the metal product dissolving in an aggressive environment.
Chemical polishing of iron
Here: https://old-rvs.itsoft.su/article/sart.html...9&conf_id=5 the influence of impurities (carbon, chromium, nickel, etc.) on the solubility of hydrogen in iron is considered. Excerpt from the text:
At 0.5% C (by mass), the absorption capacity of the alloy reaches a minimum, after which a further increase in carbon concentration is accompanied by an increase in hydrogen solubility. Alloys containing 1.5...2.0% carbon have the maximum solubility.
In pure iron, up to the melting point, the dissolution of hydrogen is accompanied by significant heat absorption. This indicates an increase in the solubility of hydrogen in the metal with increasing temperature, which is why I advised heating the parts in a vacuum (and first you need to give a vacuum and then start heating). In steels, carbon reduces the solubility of hydrogen at temperatures up to 7000C, while its diffusion mobility also decreases.
“The effect of chromium on the solubility of hydrogen in alloys with iron is different in nature in the α- and γ-phases. At temperatures of 400... 9000C, chromium slightly reduces the solubility of hydrogen in ferrite, but increases it in austenite. The same is observed in chromium and chromium-nickel steels. In chromium ferrite, the diffusion mobility decreases progressively with increasing chromium content in the iron. On the contrary, in the austenitic state, with an increase in chromium in iron, the diffusion mobility of hydrogen is not sensitive to an increase in chromium concentration. In steels with 19% (by weight) chromium at 200...6000C, a sharp decrease in the rate of hydrogen diffusion is observed. Increasing the chromium concentration to 28% has virtually no effect on the rate of hydrogen diffusion. Small additions of chromium (up to 3%) reduce the rate of hydrogen diffusion. A similar effect of chromium is observed in steels 34ХН3М and 20Х2Н4А; 40, 40X and 35X3.
The solubility of hydrogen in iron-nickel alloys increases with an increase in nickel in them to 18% (by weight) and an increase in temperature from 350 to 9000C. The diffusion mobility of hydrogen in nickel austenite does not depend on the nickel concentration. In a wider range of concentrations from 2 to 98% Ni and temperatures from 200 to 6000C, the effect of nickel on the diffusion mobility of hydrogen is complex. Nickel additions of up to 6% increase the rate of hydrogen diffusion in alloys, while large additions (up to 75%) reduce it. This effect is associated with the formation of the Ni3Fe intermetallic compound. This is also typical for low-nickel alloy steels - 34ХМ; 34ХН2М and 34ХН3М; 40Х and 40ХН. The rates of hydrogen diffusion in nickel austenite and manganese austenite are close, weakly depend on the composition and coincide with the diffusion of hydrogen in austenitic steels - 1Х18Н9Т and 30Х10Г10.
The solubility of hydrogen in silicon iron with a silicon content of up to 1.5% (by mass) increases slightly, and then at 7.8% Si it quickly decreases. At temperatures of 400... 9000C, with increasing silicon content in ferrite, the solubility of hydrogen gradually increases. The diffusion mobility of hydrogen changes little as the silicon content increases to 0.5% and decreases greatly at silicon concentrations above 7.8%.
Manganese at temperatures of 400... 9000C and its content in iron up to 35%, depending on concentration and temperature conditions, can either reduce the solubility of hydrogen or increase it. This is explained by intraphase work hardening and grain fragmentation. A sharp decrease in solubility at a manganese content of 10... 20% is associated with the formation of the ε - phase. The rate of hydrogen diffusion in carbon steels decreases as the concentration of manganese in them increases from 0.5 to 4%. Small amounts of manganese reduce the rate of hydrogen diffusion in steels - 38NSA and 35KhGSA; 40ХН and 38ХГН. Manganese increases the activation energy of hydrogen diffusion.
Preliminary deformation, mechanical and thermal treatment of structural steels have a strong effect on the solubility and diffusion mobility of hydrogen [8].
The considered effects of alloying elements and structure on the behavior of hydrogen in steels suggest ways to reduce the susceptibility of steels to hydrogen wear.
Factors that reduce the susceptibility of structural steels to hydrogen wear include the following:
1. Alloying elements in steels should not increase the solubility and diffusion mobility of hydrogen.
2. The phase composition of steel must have a minimum absorption capacity.
3. Thermal vacuum treatment of parts to remove hydrogen dissolved in the metal.
4. Application of technological processes for manufacturing parts that exclude hydrogenation.
5. Stability of the steel microstructure during operation of the friction unit.
6. High ductility of steel.
7. Fine-grained or thin-lamellar microstructure.
8. Evenly distributed soft and hard components of the structure.
If wear and tear does not play a special role, I would focus on points 1, 2, and 3.
Literature
1. Kostetsky B.I. etc. Reliability and durability of machines. Kyiv: “Technique”, 1975.
2. Cotterill P. Hydrogen fragility of metals. M.: Metallurgizdat, 1963.
3. Karpenko G.V., Kripyakevich R.I. The influence of hydrogen on the properties of steel. M.: Metallurgizdat, 1962.
4. Kolachev B.A. Hydrogen embrittlement of non-ferrous metals. M.: “Metallurgy”, 1966.
5. Geld P.V., Ryabov R.A. Hydrogen in metals and alloys, M.: “Metallurgy”, 1974.
6. Cherepanov G.P. Mechanics of brittle fracture. M.: “Science”, 1974.
7. Steklov O.I. Strength of welded structures in aggressive environments. M.:
"Mechanical Engineering", 1976.
8. Handbook of engineering materials. Steel, volume 1, ed. Yu.A. Geller. M.: “Mechanical Engineering”, 1962.
Equipment and chemicals
To work with various metals, it is necessary to select the appropriate electrolytes that will help achieve the desired result:
- Most often, compositions based on various types of acid are used - sulfuric, phosphoric or chromic.
- Glycerin can be added to increase overall viscosity if required.
- Sulfureide acts as an etch inhibitor.
- To clean various products after the procedure, various solvents or alkaline agents can be used. Formulations with surfactant active ingredients are often used.
Preparation of electrolyte for electropolishing
The preparation of electropolishing electrolyte is very simple and consists of dissolving chromic anhydride in a bath filled with a calculated amount of water, into which sulfuric and orthophosphoric acids are then gradually added in small portions (to avoid sudden heating and emission).
The solution thus obtained is heated and held at a temperature of 100 ... 110C until its density (at 20C) falls within the range of 1.72 ± 0.02 g/cm3. If for some reason such a temperature is unattainable, then to obtain an electrolyte with the required density it is treated with a current at the rate of 5 A h/l at an anode current density of 25 A/dm2.
Calculation of the amount of chemicals required to prepare 1 liter of electrolyte The amount of H3PO4 required to prepare 1 liter of electrolyte is determined by the formula:
Amount of H2SO4 for the same purposes:
Amount of chromic anhydride CrO3:
In these formulas the following designations are adopted: a1 – weight percentage of H3PO4 in the finished polishing electrolyte (see Table 1); d – density of the finished electrolyte, g/cm3; b1 – concentration of H3PO4 used, wt.%; d1 – density of H3PO4 used, g/cm3; a2 – concentration of H2SO4 (see Table 1), wt.%; b2 – concentration of H2SO4 used, wt.%; c2 – density of H2SO4 used, g/cm3; a3 – concentration of chromic anhydride (see Table 1), wt.%.
The values of b1, b2, d1, d2 are determined from reference tables.
Proportions for creating a chemical composition
Polishing is carried out in special baths. It is important to remember that their components are toxic substances and hazardous to health, especially if heating is used, so all components must be handled with the utmost care, observing the required safety precautions.
Products made of non-ferrous or ferrous metals can be processed using a universal composition that will have the necessary effect. To do this, add all the components, observing the proportions. Phosphoric acid makes up the base - 65%. Sulfuric acid should be 15% and 14% ordinary water. Chromic anhydride occupies 6%.
Advantages of electrochemical polishing
Electrochemical polishing of steel, like chemical polishing, is less labor-intensive than mechanical polishing. At the same time, this method can polish a large number of parts of various shapes and dimensions, which is impossible with manual mechanical processing with polishing wheels, which, by the way, can be made in a wide variety of ways, to suit every taste. It is important to choose the right polishing paste that ensures minimal metal removal with high quality processing. Electropolishing makes it possible to increase labor productivity, for example, the labor intensity of manual polishing for non-mechanized finishing of molds with complex profiles is 25 hours, and electropolishing is only 15 ... 25 minutes.
Polishing stainless steels, which looks especially impressive on products subjected to relief etching, is even more labor-intensive due to their viscosity. High-quality mirror polishing of stainless steel is provided by the electrochemical method.
The quality of polishing when using the electrochemical method is superior to the quality of processing by the chemical method, which ensures its primary use in the decorative finishing of bicycle parts, medical equipment, and jewelry. The effect of electropolishing is especially high in jewelry production, since it minimizes the irretrievable loss of precious metals.
The thematic scope of the article does not allow us to fully reveal the influence of other components (except for orthophosphoric and sulfuric acids) on the quality of electropolishing, but it is worth briefly dwelling on one of them - maleic anhydride. Adding it in an amount of 10 ... 20 g/l eliminates segregation and heterogeneity of the surface structure of cast steel and heat-treated parts, creates favorable conditions for the formation of a passive film, which ultimately makes it possible to obtain high-quality polished surfaces.
Advantages and disadvantages
Different types of polishing have their own characteristics, electrochemical also has pros and cons:
- This method has a beneficial effect on all the properties of steel, increasing resistance to corrosion, as well as facilitating drawing and stamping. That is why polishing of this type is often used both in laboratory research and directly for various work in industry.
- Electrochemical polishing is a cheaper and faster way to process metal products. If the mechanical method would take several hours, then using chemicals and electricity you can finish the job in a few minutes, obtaining a high-quality result.
- Electrochemical polishing is indispensable when working with complex parts that have various cavities and holes.
Chemical polishing of metals, in addition to its advantages, has some disadvantages. Almost every existing metal requires a special composition to work with it, so it is necessary to make individual solutions for different products. It is also important to choose the right ratio of components, heating temperature, current density - the quality of the result directly depends on this. Mechanical pre-grinding may be required before this treatment can be carried out. In addition, the procedure requires increased energy consumption. However, under certain conditions, the advantages of the method completely outweigh its disadvantages, allowing polishing to be carried out.
Differences between electropolishing and chemical polishing
Electropolishing, like electroplasma processing, differs from a chemical process in that an electric current is supplied through the electrolyte.
When chemical polishing, the product is lowered into a container with a chemical solution of acid or alkali. This is where the surface layer dissolves. This is accompanied by rapid boiling of the contents of the vessel. The part acquires the desired roughness in a few seconds. Unlike electropolishing, this method is less expensive. No sophisticated equipment is required here. But there are also disadvantages:
- Difficulty in controlling the progress of the process.
- Without the use of electric current, the quality of the resulting product is lower. It has no shine. Therefore, non-ferrous metal products that have a complex configuration and are not subject to high requirements are more likely to be subjected to this processing method.
Properties of metal after passaging
This procedure leads to the formation of a chemically stable layer on the metal surface that is resistant to corrosion. Passivated metal products have a longer service life. If passaging was carried out using chromates, then their surface, among other things, will have increased resistance to mechanical stress. It should be noted that the oxide layer has its own strength limit and its mechanical damage leads to the subsequent appearance of corrosion.