AD31 is part of the group of aluminum-magnesium-silicon alloys (this system is called avial). It has high ductility and, when hardened, hardness. AD31 alloy contains a small proportion of alloying elements and impurities and, due to its purity, has good electrical and thermal conductivity. Excellent anti-corrosion properties allow it to be used for the manufacture of structural elements and parts of equipment operating in difficult conditions. Can be welded, stamped and drawn to produce hollow parts of complex shapes.
Chemical composition of the alloy and its characteristics
AD31 alloy is aluminum alloyed with silicon, manganese, magnesium, titanium and chromium. The share of Al is from 97.65 to 99.35%, impurities no more than 2.35%. The chemical composition is regulated by GOST 4782-97 standards.
The introduction of alloying components into the metal composition allows one to influence the physical and mechanical properties of the final product. Iron prevents cracking of products during heat treatment. Manganese increases resistance to aggressive environments and ensures the preservation of strength under mechanical loads. Additional heat treatment gives metal products increased strength and hardness. The heat-strengthened alloy is marked AD31T1.
Aluminum alloys AD31 and AD31T1 are distinguished by good weldability and resistance to chemically active environments, sea water, and organic compounds. Products made from heat-strengthened airfoil lend themselves well to deep drawing, bending, stamping, and cutting.
Weldability of aluminum and its alloys.
Practical work No. 1
“Study of the material “Welding of aluminum””
The most important areas of application of aluminum alloys at present are aviation and rocket technology. High specific characteristics, manufacturability, and affordability are valuable properties that initially determined the choice of aircraft designers. For the operation of components under real operating conditions in space, it is also necessary that the materials be resistant to the effects of outer space factors: high vacuum, temperature changes, radiation, etc. At the moment, these requirements are best met by aluminum wrought alloys, which are used most actively.
Illustrative examples are the construction materials of the gliders of the domestic orbital ship Buran and the American Space Shuttle.
Aluminum has low strength ( σв = 8-10 kgf/mm2 ), so it is used mainly in chemical apparatus manufacturing, frame structures, for window and door frames and decorative products in construction. It has a low density of
2.7 g/cm3 , increased corrosion resistance and greater ductility
compared to low-carbon steel.
conductivity of aluminum is three times higher than that of mild steel. The melting point of pure aluminum is 658° C. When heated, aluminum easily oxidizes, forming refractory aluminum oxide ( A1203 ) , which melts at temperatures above 2060 ° C. Pure aluminum is supplied in accordance with GOST 11069-64.
In technology, not only pure aluminum is used, but also its alloys with manganese, magnesium, copper and silicon. Aluminum alloys are stronger than pure aluminum.
Alloys of aluminum with manganese, magnesium, silicon, zinc and copper have increased strength.
Aluminum and its alloys are divided into:
1.Foundry
2.Deformable (rolled, pressed, forged).
Wrought alloys are divided into:
1) Not thermally hardenable
, which include aluminum alloys with manganese and magnesium
2) Thermally hardenable
, which include alloys of aluminum with copper, zinc, and silicon.
Thermally hardened aluminum alloys have the highest strength.
For example, the mechanical properties of duralumin D16 (3.8 - 4.9% copper, 1.2 - 1.8% magnesium, 0.3 - 0.9% manganese, the rest is aluminum) are as follows: before heat treatment - σв = 22 kgf/mm2 and σ5=2%; after heat treatment - σв=42 kgf/mm2 and σ5=18%. The B95 alloy has the greatest strength among thermally hardenable aluminum alloys (σв=60 kgf/mm2, σт=55 kgf/mm2 and σ5 - about 12%), the alloy is built on the basis of aluminum - copper - magnesium - zinc.
However, thermally strengthened aluminum alloys soften during welding with a significant loss of mechanical properties. The use of these alloys for welded structures is possible only if heat treatment is provided after welding to increase the strength of welded joints.
Of the thermally non-hardening alloys, the alloys of the Al - Mg - Ti the AMg6 alloy the mechanical properties of which are as follows: σв = 32-38 kgf/mm2, σт = 16-18 kgf/mm2, σ5 = 15 - 20% and ak =3-4 kgf m/cm2. Structures made of aluminum-magnesium alloy AMg6 are manufactured mainly by welding.
In the Russian Federation, GOST 4784 “Aluminium and wrought aluminum alloys. Marks" provides marking of alloys in three ways:
Ø in alphanumeric form,
Ø only in digital form,
Ø taking into account the requirements of the international standard (international marking) ISO 209-1 (ISO 209-1 Wrought aluminum and aluminum alloys - Chemical composition and forms of products - Part 1: Chemical composition).
At the same time, the digital marking according to GOST does not coincide with the international marking of aluminum alloys.
Digital marking according to GOST indicates from left to right:
ü the first digit is the base metal (1-aluminum);
ü second digit - alloying system;
ü The third and fourth digits are the brand and modification.
alphabetic and alphanumeric markings of aluminum alloys are now widely used
For example, deformable
alloys are designated by the letters
D, AD, AK, AM, AB; foundry - AL.
The letter D denotes duralumin
D1, D16
, etc.
The letters AB stand for avial .
The letters AMg and AMts denote an alloy of aluminum with magnesium ( Mg ) and manganese ( Mts ), with the numbers following the letters AMg1; AMg6 corresponds to the approximate magnesium content of these alloys.
The letters AD correspond to deformed aluminum, the number indicates the purity of the aluminum.
The purity of alloys is indicated by the following letters after the alloy marking: Pch, Ch, Och - respectively, practically pure, pure and very pure, for impurities of iron, silicon and other controlled elements.
The condition of semi-finished products made of aluminum alloys is indicated by the following markings:
v M – soft, annealed;
v T – hardened and naturally aged;
v T1 – hardened and artificially aged;
v Н – hard-worked;
v Н1 – intensively cold-coloured (sheet cold-working ~20%), etc.
Welding technology.
For arc welding of aluminum, electrodes OZA-1 brand with a rod made of aluminum wire are used.
Welding is carried out in the lower and vertical positions with direct current of reverse polarity, with a short arc without transverse vibrations. With electrode diameter of 4 mm, the current is 120:140 A , with 5 mm - 150:170 A , and with 6 mm - 200:240 A.
Welding is carried out with heating of the product to a temperature of 200 - 250 ° C with a metal thickness of 6 - 10 mm,
300 - 350°C
at 10 - 16 mm.
Before use, the electrodes must be dried to a temperature of 200°C for 2 hours . After welding, the slag is immediately removed with a steel brush and rinsed with hot water.
To weld casting defects in products, coated aluminum electrodes of the OZA-2 brand are used.
The form of preparation of edges for welding aluminum alloys is similar to preparation for welding steels. Whenever possible, seams are made in a single pass and at high speeds.
Welding with a carbon electrode is carried out with a direct arc, direct current with straight polarity.
sheets
up to 3 mm with flanged edges without filler material. Welding thicker sheets requires cutting the edges at an angle of 60 - 75° and using an additive. It is advisable to use massive copper or steel pads under the sheets to be welded. AF-4a flux or flux of the following composition: 45% potassium chloride ; 15% lithium chloride; 30% sodium chloride ; 7% potassium fluoride and 3% sodium sulfate .
Table 1.
Approximate modes of welding aluminum with a carbon electrode
Gas welding of aluminum and its alloys ensures satisfactory quality of welded joints. The power of the gas flame during welding is selected depending on the thickness of the metal.
AF-4a flux is diluted with distilled water and applied to the welded edges and filler rod.
Table 2.
Independently study the topic: “Welding of aluminum.”
Practical work No. 1
“Study of the material “Welding of aluminum””
The most important areas of application of aluminum alloys at present are aviation and rocket technology. High specific characteristics, manufacturability, and affordability are valuable properties that initially determined the choice of aircraft designers. For the operation of components under real operating conditions in space, it is also necessary that the materials be resistant to the effects of outer space factors: high vacuum, temperature changes, radiation, etc. At the moment, these requirements are best met by aluminum wrought alloys, which are used most actively.
Illustrative examples are the construction materials of the gliders of the domestic orbital ship Buran and the American Space Shuttle.
Aluminum has low strength ( σв = 8-10 kgf/mm2 ), so it is used mainly in chemical apparatus manufacturing, frame structures, for window and door frames and decorative products in construction. It has a low density of
2.7 g/cm3 , increased corrosion resistance and greater ductility
compared to low-carbon steel.
conductivity of aluminum is three times higher than that of mild steel. The melting point of pure aluminum is 658° C. When heated, aluminum easily oxidizes, forming refractory aluminum oxide ( A1203 ) , which melts at temperatures above 2060 ° C. Pure aluminum is supplied in accordance with GOST 11069-64.
In technology, not only pure aluminum is used, but also its alloys with manganese, magnesium, copper and silicon. Aluminum alloys are stronger than pure aluminum.
Alloys of aluminum with manganese, magnesium, silicon, zinc and copper have increased strength.
Aluminum and its alloys are divided into:
1.Foundry
2.Deformable (rolled, pressed, forged).
Wrought alloys are divided into:
1) Not thermally hardenable
, which include aluminum alloys with manganese and magnesium
2) Thermally hardenable
, which include alloys of aluminum with copper, zinc, and silicon.
Thermally hardened aluminum alloys have the highest strength.
For example, the mechanical properties of duralumin D16 (3.8 - 4.9% copper, 1.2 - 1.8% magnesium, 0.3 - 0.9% manganese, the rest is aluminum) are as follows: before heat treatment - σв = 22 kgf/mm2 and σ5=2%; after heat treatment - σв=42 kgf/mm2 and σ5=18%. The B95 alloy has the greatest strength among thermally hardenable aluminum alloys (σв=60 kgf/mm2, σт=55 kgf/mm2 and σ5 - about 12%), the alloy is built on the basis of aluminum - copper - magnesium - zinc.
However, thermally strengthened aluminum alloys soften during welding with a significant loss of mechanical properties. The use of these alloys for welded structures is possible only if heat treatment is provided after welding to increase the strength of welded joints.
Of the thermally non-hardening alloys, the alloys of the Al - Mg - Ti the AMg6 alloy the mechanical properties of which are as follows: σв = 32-38 kgf/mm2, σт = 16-18 kgf/mm2, σ5 = 15 - 20% and ak =3-4 kgf m/cm2. Structures made of aluminum-magnesium alloy AMg6 are manufactured mainly by welding.
In the Russian Federation, GOST 4784 “Aluminium and wrought aluminum alloys. Marks" provides marking of alloys in three ways:
Ø in alphanumeric form,
Ø only in digital form,
Ø taking into account the requirements of the international standard (international marking) ISO 209-1 (ISO 209-1 Wrought aluminum and aluminum alloys - Chemical composition and forms of products - Part 1: Chemical composition).
At the same time, the digital marking according to GOST does not coincide with the international marking of aluminum alloys.
Digital marking according to GOST indicates from left to right:
ü the first digit is the base metal (1-aluminum);
ü second digit - alloying system;
ü The third and fourth digits are the brand and modification.
alphabetic and alphanumeric markings of aluminum alloys are now widely used
For example, deformable
alloys are designated by the letters
D, AD, AK, AM, AB; foundry - AL.
The letter D denotes duralumin
D1, D16
, etc.
The letters AB stand for avial .
The letters AMg and AMts denote an alloy of aluminum with magnesium ( Mg ) and manganese ( Mts ), with the numbers following the letters AMg1; AMg6 corresponds to the approximate magnesium content of these alloys.
The letters AD correspond to deformed aluminum, the number indicates the purity of the aluminum.
The purity of alloys is indicated by the following letters after the alloy marking: Pch, Ch, Och - respectively, practically pure, pure and very pure, for impurities of iron, silicon and other controlled elements.
The condition of semi-finished products made of aluminum alloys is indicated by the following markings:
v M – soft, annealed;
v T – hardened and naturally aged;
v T1 – hardened and artificially aged;
v Н – hard-worked;
v Н1 – intensively cold-coloured (sheet cold-working ~20%), etc.
Weldability of aluminum and its alloys.
1. Aluminum and its alloys have high thermal conductivity , heat capacity and latent heat of fusion. The thermal conductivity of aluminum is three times higher than that of low-carbon steel; when heated from 20 to 600°C, the difference in thermal conductivity increases even more. Therefore, welding of aluminum and its alloys must be performed with a relatively powerful and concentrated heat source.
2. The linear expansion coefficient of aluminum is two times
higher than the expansion coefficient of iron. This contributes to increased deformation and warping when welding aluminum products.
3. The low specific density ( 2.7 g/cm3 ) and melting point ( 660°C ) of aluminum compared to the high specific density of aluminum oxide Al2O3 ( 3.85 g/cm3 ) and its melting point ( 2050°C ) complicate the process welding Refractory and heavy oxide Al2O3 can remain in the weld metal and reduce the performance of the welded joint. Al2O3 oxide . In all cases, the surface of the metal of the product must be cleaned immediately before welding and the welding process must proceed while protecting the molten metal from the action of air gases.
There are three ways to combat aluminum oxide:
1.Welding with oxide solvent (electrode coatings, fluxes),
2.Welding without solvents, but with so-called cathode sputtering,
3.Welding with mechanical removal of oxides from the weld pool.
Solvent welding . Solvents for Al2O3 oxide and other oxides are halogen salts of alkaline earth metals ( lithium chloride, lithium fluoride, etc. ), which dissolve the oxides and, together with them, rise from the weld pool into the welding slag. Since the solution has a lower melting point, lower specific density and lower viscosity than each component separately, it is removed from the weld metal into the welding slag.
The essence of cathode sputtering is that during arc welding in argon at direct current with reverse polarity, the Al2O3 oxide film is crushed. followed by spraying oxide particles on the surface of the welded product. The thin oxide film covering the weld pool is destroyed by the impacts of heavy positive ions, the protective gas argon, formed during arc combustion. Since a positive ion has a greater mass than an electron, the resulting flow of ions is capable of crushing the oxide films of aluminum and magnesium that are created during welding. In this case, it is necessary to take into account the high speed of movement of ions, which allows sprayed oxides to exit the welding zone through a protective gas environment.
Other gases with low atomic mass (for example, 4 for helium instead of 40 for argon) are not capable of crushing and sputtering oxides.
The mechanical method of removing Al2O3 oxide from the weld pool is that the welder lowers a steel rod with a diameter of 3 - 4 mm and removes it with the oxide adhering to the surface of the rod, which is easily separated from the rod when it is shaken and lightly struck. Experienced gas or carbon arc welders often use this method without resorting to fluxes.
4. Aluminum alloys have an increased tendency to form pores. Metal porosity when welding aluminum and its alloys is caused by hydrogen, the source of which is adsorbed moisture on the surface of the base metal and especially the wire, as well as air sucked into the weld pool. In this case, aluminum in the weld pool interacts with moisture according to the reaction:
2Al+3H2O→Al2O3+6H.
To obtain pore-free welds when welding aluminum and its alloys of even small thickness, heating is sometimes required, which reduces the cooling rate of the weld pool and facilitates a more complete removal of hydrogen from the metal during slow cooling. So, for example, when surfacing on an aluminum sheet 8 mm , a pore-free seam can be obtained by heating the metal to 150°C When the metal thickness is increased to 16 mm , even heating to a temperature of 300°C does not provide pore-free seams.
However, heating sheets for welding some alloys should be used with caution. For example, when welding thick-sheet aluminum-magnesium alloys, heating to a temperature of no higher than 100 - 150 ° C . A higher heating temperature can increase the porosity of the weld due to the release of magnesium from the solid solution and the formation of hydrogen according to the reaction Mg+H2O→MgO+2H . In addition, when welding heated metal (aluminum-magnesium alloys), the mechanical properties of the welded joints are reduced.
When argon arc welding of aluminum and its alloys, pores are combated using an oxidizing atmosphere. The best results are obtained by adding 1.5% oxygen to argon. The oxidizing atmosphere in the area of the surface of the weld pool does not allow hydrogen to dissolve in the metal, so pores do not form by the end of the weld cooling.
Aluminum and its alloys are prone to overheating more than carbon steels. Therefore, welding of aluminum alloys should be carried out with less heat input, and, if possible, the weld should be performed in one pass or in two passes on both sides at high speeds.
Pros and cons of AD31
Aluminum AD31 has high performance characteristics, which significantly expands the scope of its application. Like every material, it has its advantages and disadvantages.
Pros:
- high electrical and thermal conductivity;
- pliability to all types of machining;
- lack of ferromagnetic qualities;
- corrosion resistance;
- plastic;
- ease.
Minuses:
- the need for heat treatment for use under extreme loads;
- low mechanical strength;
- brittleness when overheated.
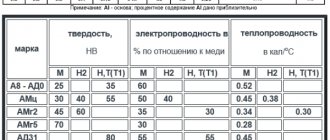
Chemical composition
Aluminum alloy AD31 is avialium. According to GOST 4784-97, it consists of 98 percent aluminum. The rest of the space is taken up by various additives of elements. The presence of magnesium in the alloy gives it strength. And silicon makes it plastic. All aluminum modifications of AD31 have beautiful and decorative properties due to the presence of aluminum in them.
You can see the full chemical composition in the table below.
Chemical composition of AD31T alloy
How to improve rental AD31
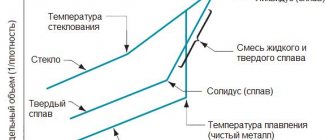
To improve the quality characteristics, the AD31 alloy is subjected to high-temperature processing. After hardening and aging, aluminum acquires new properties.
Hardening, natural and artificial aging of rolled metal, involves heating the metal to the recrystallization temperature and controlled cooling. The properties of products are affected by the time and temperature at which the cooling process occurs. Slow cooling under natural conditions makes it possible to obtain rolled metal with increased ductility. Forced cooling after hardening makes the metal harder, while its tensile strength increases by 40%.
Technological properties of AD31
Alloy AD31 is a lightweight heat-strengthened aluminum alloy of medium strength, high ductility, which is also well weldable. Airline alloys also include AD33, AD35, AB (6151). AD31 especially stands out among other alloys for its corrosion resistance. They can be welded without restrictions - all are air.
It should be noted that among other alloys, AD31 in a strengthened state has high hardness, while its electrical conductivity exceeds that of other hard alloys. Therefore, in electrical engineering it is used for the production of solid busbars with an electrical conductivity of 0.035 μOhm*m in the solid state and 0.031 μOhm*m in the normal state.
Application of AD31 alloy products
Aluminum alloy AD31 is used in the production of a wide range of rolled metal products. The most common products are:
- Circle. A rod with a round cross-section serves as a blank for bolts, nuts, screws, support fittings, and decorative elements.
- Pipe. AD31 rolled pipes are used in laying process pipelines, assembling frames, and lightweight metal structures.
- Corner. Angular steel serves as a consumable material in the construction of fences, office partitions, warehouse and commercial equipment - racks, shelving, display cases.
- Channel. Profiles with a U-shaped section, due to their low weight and spatial rigidity, are in demand in the construction of translucent structures.
- Tire. Electrical busbars are used in the installation of switchgears, busbar assemblies, and rigid conductors.
Advantages of parts made of material
To understand what advantages the alloy has, it is necessary to consider the products that are made from it.
So, the advantages include the following qualities:
- the characteristics of AD31T1 aluminum make it possible to achieve high strength structures, which at the same time will weigh quite little;
- materials have good sound insulation properties;
- service life is quite long;
- high corrosion resistance and excellent ductility;
- aesthetic appearance of products;
- ease of maintenance, which means there is no need for careful maintenance;
- ample opportunities for the production of fairly complex structural products.
However, the characteristics of the Ad31T1 aluminum alloy also have their weaknesses. Among them, it is worth highlighting that high plasticity closely borders on a high level of deformation, which becomes especially noticeable when the temperature drops significantly. This can make transporting parts more difficult.
Alloys AD31T1 and 6063
In conclusion, it is worth noting that there is an American-made analogue - alloy 6063. The main coincidence is that the two main alloying elements are silicon and magnesium. The amount of the first element can be from 0.2 to 0.6%, and the second - the same as in AD31T1: 0.45-0.9%. However, there is a slight difference, which is that 6063 uses chromium instead of titanium. In addition, the alloy belongs to the group of medium strength, but at the same time, when undergoing heat treatment, it can improve these qualities, like AD31T1.
Alloy Application
Despite the existing disadvantages, this material is used quite widely.
It is traditionally used in the production of aluminum profiles. Approximately 57% of all manufactured products are made from this alloy. They can compete well with galvanized steel, since both materials have high corrosion resistance, but aluminum alloy does not require periodic application of a protective layer, unlike steel.
Due to a number of advantages, the material is well suited for the manufacture of pipes. The characteristics of AD31T1, such as high corrosion resistance and non-toxicity, have led to the fact that the alloy has become very popular in the manufacture of containers. Typically they are then used to transport nitric acid, organic matter or even food. AD31T1 is also used to produce foil used for cans and tetrapacks.
Recently, this material has been increasingly used in the manufacture of communication cables, as well as overhead cables. This became possible due to the fact that it has a greater margin of safety than copper, which was used before. The use of AD31T1 alloy made it possible to increase the span size, as well as reduce the amount of damage during the installation of lines, which occurred quite often. As for electrical conductivity, the material took second place right after copper, but at the same time its cost is approximately 1.5 times lower. In addition, aluminum is much lighter, which plays an important role when assembling compact products that must contain a large number of current-conducting elements.