Copper belongs to the group of non-ferrous metals most widely used in industry. The serial number of copper in the periodic system of D.I. Mendeleev is 29, atomic weight A = 63.57. Copper has a face-centered cubic lattice (fcc) with a period a = 3.607 Å.
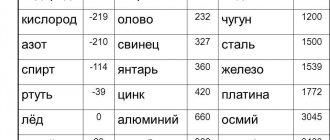
Specific gravity of copper g = 8.94 g/cm3, melting point - 1083 0C. Pure copper has high thermal and electrical conductivity. The electrical resistivity of copper is 0.0175 μΩ×m, thermal conductivity l = 395 W/(m×deg). Ultimate strength sв = 200…250 MPa, hardness 85…115 НВ, relative elongation d = 50%, relative contraction y = 75%.
Copper is a non-magnetic metal. It has good manufacturability: it can be processed by pressure, cutting, is easy to polish, is well soldered and welded, and has high corrosion resistance. The main area of application is the electrical industry.
The electrical conductivity of copper is significantly reduced in the presence of even very small amounts of impurities. Therefore, especially pure copper M00 (99.99%), electrolytic copper M0 (99.95%), and M1 (99.9%) are used as conductor materials. Technical copper grades M2 (99.7%), M3 (99.5%), M4 (99.0%).
Depending on the mechanical properties, a distinction is made between hard, cold-worked copper (MT) and soft, annealed copper (MM).
Harmful impurities in copper are bismuth, lead, sulfur and oxygen. The action of bismuth and lead is similar to the action of sulfur in steel; They form low-melting eutectics with copper, located along the grain boundaries, which leads to the destruction of copper when it is processed under pressure in a hot state (the melting point of the eutectic is 270 0C and 326 0C, respectively).
Sulfur and oxygen reduce the ductility of copper due to the formation of brittle chemical compounds Cu2O and Cu2S.
Technically pure copper is rarely used as a structural material, since it has low strength properties and hardness. The main copper-based structural materials are alloys of brass and bronze. To mark copper alloys, use the following letter designation of alloying elements:
- O - tin; C - zinc; X - chromium;
- F - iron; N - nickel; C - lead;
- K - silicon; A - aluminum; F - phosphorus;
- Mts - manganese; Mg – magnesium; B – beryllium.
History of bronze
Thanks to improvements in the processing of metals such as copper and tin, in 3000 BC. The Bronze Age began. It is characterized by the active production of an alloy such as bronze, which was used for the manufacture of tools and jewelry.
In the modern metallurgical industry, in addition to copper and tin, materials such as aluminum, phosphorus, lead, and zinc are also used. The name itself comes from the Persian word “berenj”, which translates as “copper”.
It is known that the first bronze was made from Cu and arsenic and was called arsenic. However, due to its toxicity, it was very quickly replaced by tin. It is not surprising that blacksmiths were often depicted as ugly and disfigured. In fact, this is what happened. Prolonged contact with arsenic had a very bad effect on their body. For this reason, the alloy of copper and tin is called bronze, since these are the components most often present in it.
Melting copper at home
To obtain a copper alloy at home, you need to make homemade melting equipment. The process is carried out as follows:
- The support is made of sand-lime brick.
- A metal mesh with small cells is laid on top.
- Coal is poured in and heated with a gas burner. To make the fire burn better, a stream of air is directed from the vacuum cleaner.
- A crucible with small pieces of metal is placed on the fire.
- At the end of the process, the liquid metal is poured into the mold.
The physical properties of copper alloys have made them indispensable in many areas of economic activity. Aircraft and shipbuilding cannot do without them. It is impossible to imagine watch mechanisms without such metal. Any design that has parts working in pairs needs anti-friction material.
Characteristics of bronze
We all know that a metal like copper is very soft, ductile and absolutely fragile. At the same time, it has very high electrical and thermal conductivity. An alloy of tin and copper is a material that significantly exceeds the characteristics of these chemical elements individually. In other words, bronze has high hardness and strength, but at the same time it is quite fusible.
The discovery of this alloy played a major role in the metallurgical industry. Although many other materials were invented later, even today it is very popular due to its good mechanical properties.
Form of being in nature
The appearance of crystals . The shape of the crystals is cubic, tetrahexahedral, dodecahedral, less often octahedral (possibly pseudomorphs of cuprite). The edges are often rough, with depressions or elevations. Simple crystals are rare.
Doubles.
Intergrowth twins along (111) are common, sometimes polysynthetic, often lamellar in the direction of the twin axis or elongated parallel to the diagonal twins of the plane. Typically, crystals (simple and twins) are unevenly developed: elongated, shortened or deformed. Characteristic are dendritic forms, which represent uniform accretion of many crystals (uniformly deformed or regular) in any one direction. These are, for example, twin (111) crystals, elongated along the 2nd order symmetry axis and accreted parallel to the faces of the rhombic dodecahedron) or intergrowths of regular twin crystals, branching in the direction of the edges and diagonals of octahedral faces, as well as parallel intergrowths of crystals elongated in the direction 4th order axes. In continuous deposits of native copper during etching, signs of collective crystallization are revealed with the development of large grains at the expense of smaller zonal grains of irregular shape.
Read also: Day-night sensor for turning on the light
Aggregates. Distorted crystals, in single irregular grains, dendrite-like intergrowths, thread-like, wire-like, mossy formations, thin plates, concretions, powdery accumulations and solid masses weighing up to several hundred tons.
Dendrites
Bronze's ability to resist corrosion
One of the most important properties of the alloy is its corrosion resistance. This is especially true for those compositions that contain a significant content of manganese and silicon (more than 2%).
It was found that high corrosion resistance manifests itself upon contact of bronze with water (sea and fresh), concentrated alkalis and acids, sulfates and chlorides of light metals, as well as upon contact with dry gases (tin-free bronze).
Of course, in general, the corrosion properties of the alloy depend on the alloying elements. Thus, a high lead content reduces the ability to resist corrosion, while nickel increases this property.
Chemical composition
Sometimes it contains impurities of Fe, Ag, Pb, Au, Hg, Bi, Sb, V, Ge 3 (silver copper with 3-4% Ag, ferrous copper - 2.5% Fe and golden copper - 2-3% Au). Impurities are observed more often in primary native copper; Recycled copper is usually purer. Composition of native copper from the Shamlug deposit (Armenia): Cu - 97.20 -97.46%, Fe - 0.25%; 98.3% Cu or more was determined in copper from Altai deposits.
Crystallographic characteristics
Syngony. Cubic.
Class. Hexoctahedral.
Crystal structure
The crystal structure is characterized by a face-centered lattice; Copper atoms are located at the corners and in the centers of the faces of the elementary cube. This is a formal expression of the fact that in the structure of copper there is a close packing (the so-called cubic close packing) of metal atoms with a radius of 1.27 A and a distance between nearest atoms of 2.54 A with a space fulfillment of 74.05%. Each Cu atom is surrounded by 12 similar ones (coordination number 12), located around it at the vertices of the so-called Archimedean cuboctahedron.
Main forms : a (100), d (110), o (111), l (530), e (210), h (410).
Types of bronze
Alloying elements that may be present in this alloy can significantly change its properties, and the type of bronze depends on them. In addition, tin can be replaced by other elements. For example, BrAMTS-7-1 can be deciphered as follows: 92% copper, 7% aluminum, 1% manganese. This grade of bronze does not contain tin and, due to this, has high resistance to alternating loads. It is used for the manufacture of bolts, screws, nuts and parts for hydraulic installations.
Another example is tin cast bronze of the BrO10S10 grade. It contains up to 83% copper, 9% tin, 8% lead and up to 0.1% iron, silicon, phosphorus and aluminum. It is intended for parts that operate under conditions of high specific pressures, for example, for plain bearings.
Although bronze is an alloy of tin and copper, in some cases the chemical element Sn is not used. Another example of tin-free bronze is heat-resistant. For its manufacture, only 98-99% copper and 1-2% cadmium are used. An example would be the BrKd1 brand. This is a heat-resistant cadmium bronze with high heat resistance and electrical conductivity. It can be used for the manufacture of parts for resistance welding machines, electric motor commutators and other parts operating at high temperatures and requiring good electrical conductivity.
Another type of alloy used to make gaskets in automobile bearings and bushings is pressure-processed tin bronze. The alloy of copper and tin contains alloying elements such as lead (4%), zinc (4%), aluminum (0.002%), iron (0.005%). The steel grade is called BrOTsS4-4-4. It is thanks to the percentage of these chemical elements that this alloy can be processed by pressure and cutting. The color of bronze also depends on impurities. So, the less copper the alloy contains, the less pronounced the color: more than 90% is red, up to 80% is yellow, less than 35% is steel gray.
Classification of copper alloys
Based on the nature of their interaction with copper, alloying elements and impurities are divided into three groups:
a) Elements that interact with copper to form solid solutions (Ag, Al, As, Au, Cd, Fe, Ni, Pt, P, Sb, Sn, Zn). They increase its strength, but at the same time the value of thermal and electrical conductivity decreases significantly (primarily due to the presence of antimony and arsenic).
b) Elements that are practically insoluble in copper in the solid state and form low-melting eutectics with it (Bi, Pb). The appearance of eutectics along grain boundaries leads to the destruction of copper ingots during their hot rolling (the phenomenon of red brittleness). An increased content of bismuth (more than 0.005%) causes cold brittleness of copper.
c) Elements (Se, S, O, Te) that form brittle chemical compounds with copper (for example, Cu2O, Cu2S). An increase in the sulfur content in copper, on the one hand, improves the quality of its mechanical processing (cutting), on the other hand, causes cold brittleness of copper. The presence of oxygen in copper is the cause of its “hydrogen disease”, which manifests itself in the formation of microcracks and destruction during burning (t> 400`C) in a hydrogen-containing environment. In this case, hydrogen, actively diffusing into the metal, takes oxygen away from copper oxide Cu2O with the formation of water vapor. Areas of high pressure appear in the metal, causing destruction of the material.
Alloys of copper and zinc are called brasses, tombacs (up to 10% Zn) or semi-tompacs (from 10 to 20% Zn); With the exception of alloys with nickel, all its other alloys are called bronzes.
a) Brass
Brass is a copper alloy with the addition of zinc. Zinc, the content of which can reach up to 40%, increases the strength and ductility of the alloy. The most ductile is brass, with a zinc content of about 30%. It is used for the production of wire and thin sheets.
The composition may also include iron, tin, lead, nickel, manganese and other components. They increase the corrosion resistance and mechanical properties of the alloy.
Brass can be easily processed: welded and rolled, and polished well.
A wide range of properties, low cost, ease of processing and beautiful yellow color make brass the most common copper alloy with a wide range of applications.
All brasses are divided into wrought brass, cast brass and jewelry alloys.
Deformable brass
Deformable brass can be double or multi-component.
Deformable brasses (another name is tombac) have a copper content of 90-97%. They are highly ductile, have high corrosion resistance, good anti-friction properties, and are easily welded to steel. Tompak is painted in a pleasant golden color, due to which the alloy is used for the manufacture of accessories, artistic products, and insignia.
Double deformable brass is used in the automotive industry, for the manufacture of various equipment, coils, bellows, nuts, bolts, condenser tubes, thick-walled pipes.
Multicomponent deformable brasses are used for the manufacture of parts for watches, electrical machines, ships, aircraft, and chemical equipment. They are used to produce bearing shells, fittings, bushings, springs and printing matrices.
Foundry brasses
Casting brass is used for the production of cast parts for fittings that are resistant to corrosion and high temperatures for critical parts.
Brass is marked as follows: first comes the letter L, followed by numbers indicating the percentage of copper, as well as other metals in the alloy. This marking makes it easy to navigate the properties and scope of application. For example, brasses L62 and L68 are used instead of copper for the manufacture of parts using deep stamping. The composition of brass must comply with GOST standards.
b) Bronze
BRONZE (French bronze), an alloy of copper with various chemical elements, mainly metals (tin, aluminum, beryllium, lead, cadmium, chromium, etc.). Accordingly, bronze is called tin, aluminum, beryllium, etc. The exceptions are alloys of copper and zinc, which are called brass, and alloys of copper and nickel - copper-nickel alloys.
When various elements are introduced into copper—doping—the dopant atoms increase the deformation and concentration of defects in its crystal lattice. In addition, impurity atoms interact with dislocations and impede their mobility, strengthening the copper. Therefore, the resistivity of bronzes is higher than that of pure copper, the tensile strength and hardness are also higher, and the elongation before break is lower. Bronze is better processed on metal-cutting machines and has higher casting properties than copper.
Tin bronzes
Tin bronze is the oldest alloy smelted by man. The first bronze products were produced around 3 thousand years BC. e. a smelting reducing mixture of copper and tin ores with charcoal. Much later, bronze began to be made by adding tin and other metals to copper. Bronze was used in ancient times for the production of weapons and tools (arrowheads, daggers, axes), jewelry, coins and mirrors. In the Middle Ages, large quantities of bronze were used to cast bells. Bell bronze usually contains 20% tin. Until the middle of the 19th century. For casting gun barrels, gun (gun) bronze was used - an alloy of copper with 10% tin.
Nowadays, bronze containing up to 14% tin has been used in practice. Tin bronzes have high anti-friction properties, are insensitive to overheating, frost-resistant, and non-magnetic. The main disadvantages of tin bronzes are the formation of pores in the castings, which leads to their low tightness. Tin bronzes are alloyed with zinc, lead, nickel, and phosphorus. Phosphorus forms a compound with copper, which affects the nature of crystallization processes in the alloy. It is used in tin bronze as a deoxidizing agent and removes fragile inclusions of tin oxide. When bronze contains about 1% phosphorus, it is called phosphorous. Alloying with phosphorus increases the mechanical, technological, and antifriction characteristics of tin bronzes. The introduction of nickel helps improve mechanical and anti-corrosion properties. Alloying with lead increases the density of bronzes, improves antifriction properties and machinability, but at the same time the mechanical properties are reduced. The introduction of iron helps to increase the mechanical properties of bronzes, however, with an increase in the concentration of iron, the corrosion resistance and technological properties sharply decrease.
Aluminum bronzes
Aluminum bronzes have high mechanical, anti-friction and anti-corrosion properties. To reduce shrinkage, oxidation and tendency to gas saturation, aluminum bronzes are alloyed with iron, nickel, and manganese. The main use of aluminum bronzes is for the manufacture of critical machine parts operating under intense wear and elevated temperatures.
Silicon bronzes
Silicon bronzes are characterized by high antifriction, elastic properties, and corrosion resistance. Silicon bronzes are inferior to tin bronzes in terms of shrinkage, but superior in corrosion resistance, mechanical properties and casting density. When silicon is added, an alloy is formed based on a solid solution of silicon in copper; such an alloy can be easily processed under pressure and is ductile. I use silicon bronze for the manufacture of antifriction parts, springs, membranes of devices and equipment.
Beryllium bronzes
Beryllium bronze has high mechanical strength. It is characterized by high hardness and elasticity, wear resistance and resistance to corrosive environments, which ensures the performance of products at elevated temperatures. Beryllium bronze is easy to cut and weld. Used for the manufacture of parts operated at increased speeds, loads, and temperatures.
Chrome bronzes
Chrome bronzes are distinguished by high mechanical properties, high electrical and thermal conductivity, and an elevated recrystallization temperature. These alloys are widely used for electrodes of electric welding machines and the manufacture of electric motor commutators, as higher quality alloys than cadmium bronze and collector copper used for these purposes.
c) Copper-nickel alloys
Copper-based alloys containing nickel as the main alloying element. Nickel forms a continuous series of solid solutions with copper. When nickel is added to copper, its strength and electrical resistance increase, the temperature coefficient of electrical resistance decreases, and the resistance to corrosion greatly increases. Copper-nickel alloys are well processed by hot and cold pressure.
Cupronickel
Cupronickel is a single-phase alloy, which is a solid solution; It is well processed by pressure in hot and cold conditions, after annealing it has a tensile strength of about 400 MN/m2 (40 kgf/mm2). The most valuable property of Cupronickel is its high resistance to corrosion in air, fresh and sea water. The increased nickel content, as well as the addition of iron and manganese, provide increased corrosion and cavitation resistance, especially in sea water and in a steam atmosphere.
Nickel silver
Nickel silver is an alloy of copper with 5-35% Ni and 13-45% Zn. With a high nickel content, it has a beautiful white color with a greenish or bluish tint and high resistance to corrosion. Expensive products made from nickel silver alloys called “pakfong” were brought to Europe from China in the 18th century. In the 19th century products made from alloys of this type, usually silver-plated, were produced under different names: Chinese silver, cupronickel, etc.
Bronze processing
As mentioned earlier, an alloy of tin and copper is a fairly durable material. It is difficult to sharpen, cut and press. In general, this is a casting material with low shrinkage - about one percent. And even despite its low fluidity and tendency to segregation, bronze is used for the manufacture of castings with complex configurations. Art casting is no exception.
Alloying elements that are added to the tin-copper alloy improve its properties and reduce its price. For example, alloying with lead and phosphorus improves the processing of bronze, and zinc increases its corrosion resistance. For certain purposes, deformed alloys are made. They easily change their appearance using cold forging.
Origin and location
Hydrothermal. Accumulates in placers. Nuggets weighing up to 450 tons are described as unique phenomena.
Native copper is formed under reducing conditions during various geological processes; a significant part of it is released from hydrothermal solutions. Occurs as microscopic deposits in many, predominantly mafic, igneous rocks exposed to hydrothermal solutions, such as serpentinized peridotites, dunites and serpentinites. In this case, the appearance of native copper may be associated with the decomposition of previously formed copper sulfides, for example, cubanite (Urals, Transcaucasia). A similar origin can be attributed to native copper in amphibolitized basic rocks of the Serov region of the Sverdlovsk region. In the Karabash copper gold deposit of the Chelyabinsk region, native copper is observed in vein-like bodies of diopside-garnet rocks occurring among serpentinites; native copper here is characterized by an association with cuprous gold, chalcocite, calcite, diopside, apatite, sphene, magnetite, etc. In some ancient volcanic rocks (melafyres, diabases, etc.), metamorphosed under the influence of vapors, gases and hydrothermal solutions, copper performs tonsils, forms cement between the minerals of altered lava, fills voids and cracks; accompanied by hydrothermal minerals: analcime, laumontite, prehnite, datolite, adularia, chlorite, epidote, pumpelyite, quartz, calcite. The largest deposits of this type are located on the Keweenaw Peninsula in the Lake Superior region (Michigan, USA), where mineralization is confined to the Upper Proterozoic strata. The bulk of copper is mined from melafires and conglomerates, but the largest copper deposits (up to 400 tons or more) are found in calcite veins containing native silver and domeicite.
Copper nugget
Application area
Of course, the use of bronze does not lose its popularity in our time. Souvenir products, decorative interior items, decorations for gates and gates... In addition, the alloy is used for the manufacture of fittings (handles, hinges, locks) and plumbing fixtures (taps, fittings, gaskets, faucets). Bronze also has wide range of uses in industrial applications. Thus, cast alloy is used for the manufacture of bearings, sealing rings, and bushings.
The widespread use of bronze is particularly influenced by its corrosion properties. For this reason, it is used to make parts of mechanisms that operate in constant contact with water. The high elasticity of the alloy makes it possible to make springs and parts of control and measuring equipment from it.
Physical properties
Optical
The color of a fresh fracture is light pink, quickly turning into copper-red, then brown; often with yellow or mottled tarnish.
The streak is copper-red, shiny.
Transparency. Opaque. In the thinnest plates it shines through in green.
Mechanical
No cleavage is observed.
The fracture is splintered and hooked.
Chemical properties
Easily dissolves in dilute HNO 3 and aqua regia, in H 2 SO 4 - when heated, in HCl - with difficulty. Ammonia dissolves in an aqueous solution, turning it blue. In polished sections it is etched with all the main reagents. The internal structure is easily revealed using NH 4 OH + H 2 O 2 or HCl + CrO 3 (50% solution).
Other properties
Very malleable and viscous. Electrical conductivity is very high; is significantly reduced by impurities.
Heating behavior. Pure copper melts at 1083°. Thermal conductivity is slightly less than that of silver.
Bronze remelting
Of course, each alloy has both its pros and cons. Bronze is an alloy that consists of copper and tin, and therefore it withstands any melting. It can be used several times for completely different purposes. On the other hand, if bronze contains a large amount of impurities, such as magnesium, silicon, aluminum, then during remelting the mechanical properties may decrease.
This is due to the fact that alloying elements that improve the characteristics of bronze are oxidized during melting and form refractory oxides, which are located along the boundaries of the crystal lattice. They break the bond between grains, which makes bronze more brittle.
Sources of copper for recycling
Saving resources is an important environmental and technological task. Copper is too valuable an element to be thrown away easily. Therefore, when disposing of household devices and appliances (TVs, refrigerators, computer equipment), you need to cut off all copper-containing elements and take them to recycling collection points. Factories must organize a centralized collection of decommissioned power cables and transformers, electric motors, and other copper-containing parts and devices. There is a certain copper content in spoiled fluorescent lamps, which should also be taken into account when recycling.
Read also: Galvanic kitchen what is it
Copper and copper alloys, mastered by mankind at the very dawn of civilization, remain popular materials in the technological era, the basis of which is iron. Modern industrial production cannot be imagined without the use of non-ferrous metals. In the future, the need for copper and its alloys will only grow, so it is very important to treat these materials sparingly and use them rationally.
Copper belongs to the group of non-ferrous metals most widely used in industry. The serial number of copper in the periodic system of D.I. Mendeleev is 29, atomic weight A = 63.57. Copper has a face-centered cubic lattice (fcc) with a period a = 3.607 Å. The specific gravity of copper is g = 8.94 g/cm 3, the melting point is 1083 0 C. Pure copper has high thermal and electrical conductivity. The electrical resistivity of copper is 0.0175 μΩ×m, thermal conductivity l = 395 W/(m×deg). Ultimate strength sв = 200…250 MPa, hardness 85…115 НВ, relative elongation d = 50%, relative contraction y = 75%.
Copper is a non-magnetic metal. It has good manufacturability: it can be processed by pressure, cutting, is easy to polish, is well soldered and welded, and has high corrosion resistance. The main area of application is the electrical industry.
The electrical conductivity of copper is significantly reduced in the presence of even very small amounts of impurities. Therefore, especially pure copper M00 (99.99%), electrolytic copper M0 (99.95%), and M1 (99.9%) are used as conductor materials. Technical copper grades M2 (99.7%), M3 (99.5%), M4 (99.0%).
Depending on the mechanical properties, a distinction is made between hard, cold-worked copper (MT) and soft, annealed copper (MM).
Harmful impurities in copper are bismuth, lead, sulfur and oxygen. The action of bismuth and lead is similar to the action of sulfur in steel; They form low-melting eutectics with copper, located along the grain boundaries, which leads to the destruction of copper when it is processed under pressure in a hot state (the melting point of the eutectic is 270 0 C and 326 0 C, respectively).
Sulfur and oxygen reduce the ductility of copper due to the formation of brittle chemical compounds Cu2O and Cu2S.
Technically pure copper is rarely used as a structural material, since it has low strength properties and hardness. The main copper-based structural materials are alloys of brass and bronze. To mark copper alloys, use the following letter designation of alloying elements:
- O - tin; C - zinc; X - chromium;
- F - iron; N - nickel; C - lead;
- K - silicon; A - aluminum; F - phosphorus;
- Mts - manganese; Mg – magnesium; B – beryllium.
How to distinguish bronze from brass and copper
One of the most common questions is the difference between this alloy and others that are similar in appearance. Of course, this is quite easy to do within industry and with the help of special reagents. But what if you need to determine the material at home?
To begin with, the alloy consists of tin and copper. The percentages of these substances may vary. The more copper, the brighter the color, but due to the tin content in the alloy, it will be an order of magnitude heavier than, for example, pure Cu.
If we compare bronze with brass, the latter has a more yellowish tint. Copper itself is very ductile, but alloys based on it are quite elastic and hard. You can also determine what material is in front of you by heating. Thus, when exposed to high temperatures, brass releases zinc oxide and the product acquires an ashy “patina.” But bronze will not change its properties when heated.
Physico-chemical properties of copper
In its natural environment (in air), copper has a bright yellow-red hue. This color is given to the metal by the oxide film that forms on its surface. Pure metal is a fairly soft material; it can be easily rolled and drawn. But the use of certain impurities in its production makes it possible to increase its hardness and change other parameters.
The density of this material is 8890 kg/m3, the melting point is within 1100 °C.
The key property that determined its applicability in everyday life and production. In addition to high electrical conductivity, copper is characterized by high thermal conductivity. The use of impurities such as iron, tin and some others have a significant impact on its properties.
In addition to the above parameters, copper has a high melting and boiling point. Copper is highly resistant to corrosion.
Copper in nature
The physical parameters of copper make it possible to obtain various products from it, for example, wire several microns thick.
Copper and its compounds have found their application, first of all, in the electrical industry, although any other area of industry is unlikely to survive without it.
Works of art
Quite often you can find various bronze figurines and figurines. Many works of art were created in ancient times and the Middle Ages.
Alloys containing copper and tin are used for the manufacture of:
- Fences and gates that are not only incredibly beautiful, but also durable.
- Elements of staircase structures.
- Souvenir products and sculptural compositions.
- Decorative lighting fixtures: sconces and chandeliers.
- Items for interior decoration.
In order to cast the required composition, a special model is created from wood, plaster or polymer materials - the so-called molding. The cavities of this figure are filled with clay and removed after casting. After manufacturing, the surface can be covered with gold, a layer of nickel, chromium or silver.
It is very important to note that, as a rule, an alloy of tin and copper without alloying elements is used to make works of art. This is due to the fact that the more such components are present in bronze, the greater its shrinkage, which negatively affects the quality and shape of the product.
Copper production methods
Among the methods for producing copper from ores with concentrates, the pyrometallurgical method and the hydrometallurgical method are distinguished. The latter is not widely used. This is dictated by the impossibility of simultaneous reduction of other metals with copper. It is used to process oxidized or native ore with low copper content. Differing from it, the pyrometallurgical method allows the development of any raw material with the extraction of all components. It is very effective for ores undergoing beneficiation.
The main operation of this copper production process is smelting. In its production, copper ores or their roasted concentrates are used. In preparation for this operation, the copper production scheme provides for their enrichment by flotation. At the same time, ores containing valuable elements along with copper: tellurium or selenium, gold and silver, should be enriched in order to simultaneously transfer these elements into copper concentrate. The concentrate formed by this method can contain up to 35% copper, the same amount of iron, up to 50% sulfur, as well as waste rock. It is roasted in order to reduce its sulfur content to an acceptable level.
The concentrate is fired in a predominantly oxidizing environment, which removes approximately half of the sulfur content. The concentrate obtained in this way, when melted, gives a fairly rich matte. Firing also helps to halve the fuel consumption of a reverberatory furnace. This is achieved by high-quality mixing of the mixture composition, ensuring its heating to 600ºС. But it is better to process copper-rich concentrates without burning them, since after this the loss of copper with dust and in slag increases.
The result of this sequence of copper production is the division of the melt volume in two: matte-alloy and slag-alloy. The first liquid, as a rule, consists of copper and iron sulfides, the second - oxides of silicon, iron, aluminum and calcium. The processing of concentrates into matte alloy is carried out using electric or reverberatory furnaces of various types. Pure copper or sulfur ores are best smelted using shaft furnaces. For the latter, it is also worth applying copper-sulfur fusion, which allows you to capture gases while simultaneously extracting sulfur.
Copper ores with coke, as well as limestones and recycled products are loaded into a special furnace in small portions. The upper part of the furnace creates a reducing atmosphere, the lower part – an oxidizing one. As the lower layer melts, the mass slowly descends to meet the heated gases. The upper part of the furnace is heated to 450 ºС, and the temperature of the exhaust gases is 1500 ºС. This is necessary when creating conditions for purification from dust even before the release of vapors with sulfur begins.
As a result of such smelting, matte is obtained, including from 8 to 15% copper, slag, mainly containing lime with iron silicate, and also blast furnace gas. Sulfur is removed from the latter after preliminary dust precipitation. The problem of increasing the percentage of Cu in matte alloy in copper production in the world is solved by using contractile smelting. It consists of placing coke, quartz flux, and limestone in a furnace along with matte.
When the mixture is heated, the process of reduction of copper oxides and iron oxides occurs. Iron and copper sulfides fused with each other make up the original matte. The molten iron silicate, when flowing along the surfaces of the slopes, is absorbed by other components, replenishing the slag. The result of such smelting is the production of enriched matte with slag, including copper up to 40% and 0.8%, respectively. Precious metals, such as silver and gold, hardly dissolving in the slag alloy, end up entirely in the matte alloy.