Influence of alloying additives
Metals in compositions improve and change the physical and chemical properties of the base metal. The main emphasis is on improving mechanical performance. Aluminum improves the overall structure, casting properties, and increases strength. Zinc also increases strength and helps reduce grain in the casting. The main purpose of introducing manganese, in addition to increasing strength, is to increase chemical resistance to aggressive environments and reduce the harmful effects of iron impurities.
Rare earth metals, despite their small quantities, greatly change the chemical and physical properties, increasing heat resistance, improving ductility, malleability by reducing grains and changes in the crystal lattice.
The addition of zirconium reduces the solubility of hydrogen in the melt, which in the pure composition is significant. By binding hydrogen, zirconium also helps reduce the porosity and graininess of castings.
The introduction of lithium into some compositions makes it possible to obtain magnesium alloys with a record low density - 2 times less than that of aluminum, while maintaining high strength and ease of machining. These alloys are most widely used in the aerospace industry, where reducing the overall weight of the structure increases the payload mass.
Appearance of magnesium alloys
Some metals, on the contrary, are undesirable even in small quantities. Thus, impurities of iron or nickel, even in a volume of thousandths of a percent, sharply reduce the corrosion resistance of the alloy. Dissolved hydrogen increases the porosity of the material, causing grains to enlarge, thus reducing the strength of the product.
Characteristics of magnesium
Industrial production and use of magnesium began relatively recently - only about 100 years ago. This metal has a low mass, as it has a relatively low density (1.74 g/cmᶟ), good stability in air, alkalis, gaseous environments containing fluorine and in mineral oils.
Its melting point is 650 degrees. It is characterized by high chemical activity up to spontaneous combustion in air. The tensile strength of pure magnesium is 190 MPa, the elastic modulus is 4,500 MPa, and the relative elongation is 18%. The metal has a high damping capacity (effectively absorbs elastic vibrations), which provides it with excellent tolerance to shock loads and reduced sensitivity to resonance phenomena.
Other features of this element include good thermal conductivity, low ability to absorb thermal neutrons and interact with nuclear fuel. Thanks to the combination of these properties, magnesium is an ideal material for creating hermetically sealed shells for high-temperature elements of nuclear reactors.
Magnesium alloys well with various metals and is one of the strong reducing agents, without which the process of metallothermy is impossible.
In its pure form, it is mainly used as an alloying additive in alloys with aluminum, titanium and some other chemical elements. In ferrous metallurgy, with the help of magnesium, deep desulfurization of steel and cast iron is carried out, and the properties of the latter are also improved through spheroidization of graphite.
Main types of magnesium alloys
Magnesium alloys differ in their manufacturing technology. In accordance with this, the following classification has been adopted for all compositions with magnesium:
- casting magnesium alloys, which have high casting properties;
- wrought alloys that can be easily machined by pressing forging
The chemical composition of the additives is selected in such a way as to minimize the subsequent processing of cast alloys and increase the processability of wrought alloys.
Within each group, materials are divided according to their properties, casting method, processing methods (pressing, forging, stamping and rolling).
Each of the two listed groups includes compositions with different strength, heat resistance, chemical resistance, as well as different welding abilities.
History of discovery
Magnesium (Mg) was discovered by scientists as a result of random experiments in 1695. Chemical scientists have isolated bitter salt from the healing waters of the Epsom natural spring, which is located in the UK. The resulting salt had the effect of a mild laxative on the human body, and was prescribed for appropriate purposes for a long time. This substance was originally called “magnesia”.
In 1798, a little-known chemist Anton von Ruprecht managed to isolate a new element from “bitter salt” using the method of reduction with charcoal, designating it “Austrian”. After some time, it was possible to find out that “Austria” is actually magnesium with an abundance of ferrous impurities.
The next chain of magnesium discoveries followed at the beginning of the 19th century. In 1808, the British chemist Humphry Davy obtained an aggregation of a new metal by electrolysis of a wet mixture of magnesia and mercuric oxide, calling it “magnesium”. It is noteworthy that this name is still accepted in some countries.
In 1829, the French chemist A. Bussy isolated magnesium by reducing molten chloride with potassium metal. In 1830, M. Faraday managed to extract magnesium by electrolysis of magnesium chloride heated to the melting point.
Markings and properties
The domestic industry labels magnesium alloys based on two-letter markings with additional numbers:
- foundry - ML1 - ML20;
- deformable - MA1 - MA19;
- heat-resistant magnesium alloys VML1 – VML2.
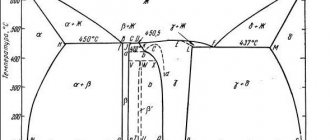
Casting alloys are mostly produced on the basis of the Mg – Al – Zn system, which is a solid solution of aluminum and zinc in magnesium. The best casting properties are found in such types of solutions as grades ML4 – ML6. These alloys have high fluidity, low shrinkage and are not prone to the formation of cavities. Such characteristics allow the use of these brands for precision casting of workpieces of any shape and size.
Heat-resistant alloys, which also include grades ML9 - ML14, are capable of withstanding temperatures up to 350 ˚C for a long time and up to 400 ˚C for a short time. The composition is based on the Mg – Zn system with the addition of zirconium. In addition to heat resistance, these alloys withstand static and fatigue loads well.
Additional alloying with rare earth metals in some formulations can reduce the likelihood of cracking, which increases resistance to deforming loads.
Deformed alloys are produced on the basis of the Mg – Al, Mg – Zn, Mg – Mn systems. Aluminum and zinc contribute to increased ductility and allow castings to be subjected to pressure processes such as forging, pressing, stamping, as well as cold and hot rolling.
Like castings, wrought ones are additionally alloyed with rare earth metals, but other materials have also been found here. These include cadmium and silver, which increase strength while increasing ductility.
Grades MA11 - MA12 of wrought magnesium alloys are heat-resistant materials, like similar casting ones.
Alloys MA14 and MA19 are characterized by the fact that they do not allow the use of welding during further use, unlike most other compositions.
Magnesium and its alloys
Magnesium is a silver-white metal; specific gravity 1.74; melting point 650°; magnesium crystallizes in the hexagonal system. Pure magnesium is quite stable in air (almost as good as aluminum). A solution of table salt, sea water, acids (except hydrochloric acid) quickly destroy magnesium; Magnesium is resistant to alkalis. When burned, magnesium produces a bright white light; Magnesium is 4 times lighter than iron, which is why its alloys are called ultra-light.
Due to the fact that the mechanical properties of pure magnesium are low, it is therefore not used in its pure form as a structural material.
Chemical properties of magnesium. The chemical properties of magnesium are quite peculiar. It easily removes oxygen and chlorine from most elements, and is not afraid of caustic alkalis, soda, kerosene, gasoline and mineral oils. Magnesium almost does not interact with cold water, but when heated, it decomposes with the release of hydrogen. In this respect, it occupies an intermediate position between beryllium, which does not react with water at all, and calcium, which easily interacts with it. The reaction is especially intense with water vapor heated above 380 °C.
Based on density, magnesium alloys are divided into light and ultra-light . Ultra-light alloys include alloys alloyed with lithium ( MA21, MA18 ), and light alloys include all the rest. Magnesium alloys with lithium ( MA21, MA18 ) are the lightest structural metal materials.
When classified according to possible operating temperatures, magnesium alloys are divided into the following groups:
- designed to operate at normal temperatures (general purpose alloys);
- heat-resistant (for long-term operation at temperatures up to 200°C);
- highly heat-resistant (for long-term operation at temperatures up to 250 – 300°C)
- Designed for use at cryogenic temperatures.
There are thermally hardenable and non-thermally hardenable alloys.
Classification and characteristics of magnesium alloys
The properties of magnesium are significantly improved by alloying. Magnesium alloys are characterized by low density, high specific strength, and the ability to absorb vibration well. The strength of alloys with appropriate alloying and heat treatment can reach 350-400 MPa. The advantage of magnesium alloys is their good machinability and weldability.
The disadvantages of magnesium alloys are poor casting properties and a tendency to gas saturation, oxidation and ignition during casting. To prevent defects during smelting, special fluxes are used, small additions of calcium (0.2%) are used to reduce porosity, and beryllium additives (0.02-0.05%) are used to reduce oxidation. In addition, less corrosion resistance than aluminum alloys, difficulty in smelting and casting, and the need for heat during forming.
On the other hand, elements such as manganese, zirconium, zinc, titanium improve the corrosion resistance of magnesium: when adding a few ninth percent of titanium to a magnesium alloy, the corrosion resistance increases by 3 times.
The main strengthening alloying elements in magnesium alloys are aluminum and zinc. Manganese has little effect on strength properties. It is introduced mainly to increase corrosion resistance and grain refinement.
Heat treatment of magnesium and aluminum alloys has much in common. This is explained by similar melting temperatures and the absence of polymorphic transformations.
To increase strength properties, magnesium alloys are subjected to hardening and aging . Due to the low diffusion rate, hardening is usually carried out in air; artificial aging is used at relatively high temperatures ( up to 200–250 °C ) and longer exposures ( 16–24 hours ).
The strength characteristics of magnesium alloys increase significantly during thermomechanical treatment, which consists of plastic deformation of the hardened alloy before aging.
Magnesium alloys have high ductility when hot and are easily deformed when heated. For deformed alloys, diffusion annealing is usually combined with heating for forming. Magnesium alloys are easy to cut and are easy to grind and polish. They are welded satisfactorily by resistance roller and arc welding, which is recommended to be carried out in a protective atmosphere (Fig. 1).
Rice. 1. Welding magnesium alloys
Magnesium parts absorb vibration very well . Their specific vibration strength is almost 100 times greater than that of the best aluminum alloys, and 20 times greater than that of alloy steel. This is a very important property when creating a variety of vehicles.
Magnesium alloys are superior to steel and aluminum in specific rigidity and are therefore used for the manufacture of parts subject to bending loads (longitudinal and transverse). Magnesium alloys are non-magnetic , do not produce sparks at all upon impact and friction, and are easily machined by cutting (6–7 times lighter than steel, 2–2.5 times lighter than aluminum).
Magnesium and its alloys have very high cold resistance.
The possibilities for using magnesium are far from being exhausted, and given the wide distribution of magnesium in nature, the relative simplicity of the methods for its production and a number of favorable properties of this metal, we can assume that the further development of magnesium metallurgy will be primarily determined by its general technical significance.
sensitive to strain rate than aluminum and copper alloys . An increase in the deformation rate during stamping of magnesium alloys leads to a significant narrowing of the permissible temperature range. In this regard, it is recommended to deform forging magnesium alloys using hydraulic or crank presses at reduced deformation rates. Recommended temperature ranges for stamping some grades of magnesium alloys are given in Table 1 (Fig. 2). Chemical composition and mechanical properties of some domestic magnesium alloys (Table 2).
Table 1. Recommended temperature ranges for stamping some grades of magnesium alloys
Rice. 2. Sequence of operations for obtaining a workpiece from a magnesium alloy
Table 2. Chemical composition and mechanical properties of domestic magnesium alloys
Basically, wrought magnesium alloys are used in the form of rods and shaped profiles for the manufacture of parts by hot stamping (Fig. 3). To improve their ductility, pressure treatment is carried out at temperatures of 350-450 ° C , since the hexagonal lattice of magnesium makes it difficult for them to deform at room temperature.
Rice. 3. Products made of magnesium alloys
The most durable wrought alloys are alloys of magnesium with aluminum (MA5) and magnesium with zinc, additionally alloyed with zirconium (MA14, an analogue of the American alloy ZK60A), cadmium, rare earth metals and other elements (MA15, MA19, etc.).
Aluminum and zinc are effective solution strengtheners. However, their concentration should not exceed 10 and 6%, respectively. With a higher content of these elements, the plasticity decreases sharply. The appearance of strengthening phases Mg4Al3 and MgZn2 in the structure during aging provides additional strengthening. Zirconium refines the grain, while cadmium and rare earth elements simultaneously increase both strength and ductility.
The tensile strength of high-strength magnesium alloys after heat treatment is about 350 MPa . The relatively small strengthening effect is explained by the tendency of the strengthening intermetallic phases to coagulate during the decomposition of the solid solution.
Alloy MA1, containing about 2% MC without other components, is characterized by high ductility and is used as sheet material. The lightest structural materials are magnesium alloys with lithium (MA18, MA21). The density of the MA18 alloy (analogous to the American alloy LA91) is 1.3-1.65 g/cm3. Magnesium-lithium alloys have increased ductility and toughness and can be processed by cold pressure. These alloys are easily weldable and have satisfactory corrosion resistance.
Cast magnesium alloys
Cast magnesium alloys are close in chemical and phase composition to wrought ones.
Compared to deformable parts, cast parts can significantly save metal. High dimensional accuracy and good surface quality make it possible to virtually eliminate machining operations. The disadvantage of cast magnesium alloys is their lower mechanical properties due to the coarse-grained structure and shrinkage porosity associated with a relatively wide crystallization range.
To increase strength and modification, calcium and zirconium are introduced. Additional alloying with cadmium increases the level of mechanical and technological properties.
The most common magnesium casting alloy is MJI5, which is characterized by good fluidity, low porosity and good machinability. Castings from this alloy are produced by earth casting, metal molds and pressure casting. It is used for the production of large-sized castings of engine crankcases, instrument housings, pumps, and gearboxes for cars and aircraft (Fig. 4).
To reduce the weight of parts, magnesium alloys alloyed with 12-13% lithium are used. Their fluidity is at the level of the ML5 alloy. Mg-Li alloys do not tend to form hot cracks.
Rice. 4. Cast magnesium alloy products
Application of magnesium alloys
(Fig. 5)
Due to their low density and high specific strength, magnesium alloys are widely used in aircraft construction. Instrument housings, pumps, lights and cabin doors are made from them. The fuselages of helicopters from Sikorsky (USA) are almost entirely made of magnesium alloys. In rocket technology, magnesium alloys are used to make rocket bodies, fairings, stabilizers, and fuel tanks. The heat capacity of magnesium is approximately 2.5 times greater than that of steel. Having absorbed the same amount of heat, it will heat up 2.5 times less. In a short flight, magnesium alloys do not have time to overheat, despite their low melting point. In short-term air-to-air missiles and guided missiles, magnesium alloys make up the bulk of the structure. The use of magnesium alloys made it possible to reduce the mass of missiles by 20-30%.
Brackets, fastening elements, ailerons, tail parts are made from cast alloys; hull skins, stringers, spars, support structures for brakes, wave guides and other parts are made from deformable alloys.
Magnesium alloys are used in transport engineering for the manufacture of engine crankcases and car gearboxes.
Magnesium alloys are used in the construction of portable hand and mechanized tools and machines (drilling and grinding machines, saws for the forestry industry, lawn mowers, pneumatic tools, etc.).
They are used in electrical and radio engineering (instrument housings, electric motors), in the textile industry (bobbins, bobbins, reels, etc.) and other industries.
Due to their low resistance to corrosion, products made from magnesium alloys are subjected to oxidation. Paint and varnish coatings are additionally applied to the oxidized surface.
An important area of application of magnesium is nuclear energy. Due to the ability to absorb thermal neutrons, the absence of interaction with uranium and good thermal conductivity, magnesium alloys are used to make shells of fuel elements in nuclear reactors.
The high electronegative potential of magnesium alloys allows them to be used for tread protection against sea corrosion of ships and structures operating in sea water, and for protection against underground corrosion of gas pipelines, oil pipelines, etc.
Rice. 5. Products made of magnesium alloys
315
Receipt and production
For the manufacture of alloys, materials of high purity are used, since, as mentioned above, even the smallest impurities of undesirable elements can significantly deteriorate the properties of the finished product.
The production of magnesium alloys is facilitated by the fact that the melting temperature of the melt does not exceed 700˚C. To obtain a material with the required properties, the required amount of alloying elements is introduced into the pure magnesium melt. The gas composition of the atmosphere around the melt must be cleared of hydrogen, since its high solubility in magnesium can lead to defects in the internal structure.
Minerals, deposits
Our hero is so chemically active (simply “promiscuous”) that it is almost impossible to find it in nature in its pure form.
Natural sources of magnesium - minerals:
- brucite;
- kieserite;
- dolomite;
- magnesite;
- bischofite;
- epsomite;
- carnallite.
Fire metal can even be extracted from sea water. Self-sedimented lakes (the water in them is called brine) contain a large amount of mineral salts, including magnesium.
Educational: there are many such lakes in the Astrakhan region. These are Belinsky, Zinzilinsky, Mochagovsky self-sedating lakes (the list goes on).
The largest Russian group of deposits - Satkinskoye - (14 explored) is located in the Chelyabinsk region, near the city of Satka. High purity magnesium ores are concentrated here.
We recommend: NICKEL - the “stepson” in the family of silver metals
Casting processing
The mechanical properties of magnesium-based castings can be improved by using several techniques:
- homogenization (hardening);
- hardening with aging to stabilize properties;
- recrystallization annealing to relieve mechanical stress after pressure treatment;
- diffusion annealing to align the internal structure and chemical composition of metal grains.
Aluminum-magnesium alloy castings
It should be noted that for most alloys, after heat treatment, the mechanical strength does not increase.
Magnesium in our life
Metal and its alloys have found wide application in various spheres of life.
- The ability of metal to produce a bright fire was used at the dawn of photography.
- The lightness of the metal opened the way for him to aviation. Our laptops and many cameras contain magnesium parts - don’t carry a heavy device if you can make it lightweight.
- In chemical current sources, the energy of chemical reactions is directly converted into electrical energy. Pure metal and its compounds in electric batteries give them high EMF and excellent energy characteristics.
Magnesium metal
The anode in such batteries is magnesium. The following is used as a cathode:
- manganese;
- bismuth;
- sulfur;
- silver chloride;
- lead chloride mixed with graphite;
- manganese dioxide with graphite.
We recommend: IRON - the No. 1 metal in the world
Refractory materials are necessary for the lining of metallurgical furnaces and crucibles.
Cheap and high-quality raw materials for this can be magnesium minerals:
- bischofite;
- magnesite;
- dolomite.
In warfare, magnesium “illuminates dark places.” Or more simply, it is used to make light-sound and flash-noise ammunition (cartridges, grenades, shells). It won't kill you completely, but it will stun and disorient you.
They are used in anti-terrorist operations, during the release of hostages, and the dispersal of illegal gatherings (during mass riots).
Incendiary bombs, tracer bullets, flares and flares - brightly burning metal is used everywhere.
Magnesium preparations are necessary in medicine. A lack of macronutrients is detrimental to the cardiovascular system. Coronary artery disease, arterial hypertension, arrhythmias - each of these diseases is aggravated by magnesium deficiency.
The lack of a few grams of metal has a bad effect on our nerves (depression, migraines, dizziness, anxiety, irritability).
Important: everyone’s need for magnesium increases under stress and physical activity; in athletes - during grueling training and competitions.
Toyota specialists have developed a battery (based on sulfur-magnesium cells). The battery performance is enviable. The catch is that self-discharge occurs in the battery (the cathode is electrochemically reduced, polysulfide anions are formed and pass into solution). Until this problem is solved, specialists are only dreaming of sulfur-magnesium batteries.
Magnesium metal has strong reducing properties. It is used to produce beryllium, vanadium, and chromium. The metal is used as an alloy in steels and cast iron.
Organomagnesium compounds are increasingly used in the chemical synthesis of halogen derivatives, alcohols, and hydrocarbons.
Where else are magnesium compounds used?
Colorless crystals of magnesium fluoride are used in special optics (the substance is transparent in the range from ultraviolet to infrared.
Magnesium stearate is a food additive E470. Used in the cosmetics, food and pharmaceutical industries.
Magnesium alloys are used in the manufacture of jackhammers, in the nuclear and oil industries.
Getting magnesium
There are two technologies used in industry for producing this metal:
- electrolytic;
- heat treatment of raw materials.
The first option requires mixing dehydrated sodium, magnesium and potassium chlorides in an electrolytic bath. When melted, magnesium is reduced. The resulting metal is drained and the bath is filled again with the raw material mixture. At the output, magnesium contains about 2% impurities. If necessary, it is easy to clean it further, achieving an almost ideal indicator. Clean magnesium immediately before it cools down.
The second method of obtaining magnesium involves using dolomite or salt (lake or sea) water as the starting material. Dolomite will need to be mixed with coke and heated to an operating temperature of at least 2100 degrees. The process plant provides for the removal and condensation of magnesium vapor.
If magnesium alloys are so amazing, where have they been all this time?
The Samsung NX1 camera has a body made of magnesium alloy.
Historically, aluminum has gained popularity faster, as the metal is great for everything from soda cans to car engines. It was light, strong, recyclable, and improved technology made it cheap. The commercial use of magnesium began much later, but the material's popularity is now on the rise as its cost-effectiveness approaches that of aluminum. On the one hand, magnesium raw materials are still much more expensive than aluminum, but on the other hand, it is easier for machines to make into an alloy, so it is cost-effective, just like aluminum.