The damage caused by such a process is enormous. Sometimes the cost of the damage it causes is many times greater than the cost of producing the metal itself and the subsequent use of parts made from it. According to world statistics, every sixth blast furnace in the world works to cover the consequences of this phenomenon.
Corrosion is the process of natural destruction of metal under the influence of environmental factors in which it is located. The name of the phenomenon itself is taken from Latin. Corrosion means corroding.
Electrochemical corrosion rate.
Thermodynamics establishes the possibility of
the corrosion process occurring, but does not give real ideas about the corrosion rate.
2) Depth indicator, Kg - reduction in metal thickness per unit of time, [mm/year].
There are tables for converting units.
There are 3 groups of metals based on corrosion rate:
a) 1.5 mm/year – not durable , not used in corrosive environments.
3) Corrosion current density (or corrosion current), i
cor (
Icor
) - the amount of current per unit area of metal dissolved per unit time.
According to Faraday's law: ikor = m F/Me S t,
where M
E is the molar mass of the metal equivalent, g/mol;
F – Faraday number, 96500 cells/mol;
i
cor—corrosion current density, A/m 2 ;
Km, Kg, icor - direct indicators of corrosion.
Ekor
- stationary potential of the corroding metal (installed on the surface of the metal), at which conjugate reactions of Me ionization and Ox reduction occur.
for Fe
corrosion rate:
Km
= 1 g/(m 2 × day) and
i
corr = 0.04 A/m 2
When the CGE operates, polarization occurs, leading to a decrease in EMF and a decrease in V corrosion. The rate of electrochemical corrosion depends on the rate of the limiting stage: V=Vlim.st. (anodic dissolution of M; cathodic absorption of O2 - most often; cathodic release of H2). In order for ↓V to be ↓Vlim.st., i.e. ↑ polarization. Polarization curves of a corrosive microelement - corrosion diagrams .
Date added: 2014-12-03; ; ORDER A WORK WRITING
Source
Durability of a metal screw pile taking into account corrosion processes in the soil
One of the most significant issues that arise when using metal structures in construction is the issue of the resistance of such structures to corrosion processes and the associated durability of buildings and structures.
Currently, there is a set of interconnected interstate standards that establish general requirements, rules, norms and methods for protecting products, structures and materials from corrosion, aging and biological damage at all stages of the life cycle of products and structures, research and justification for development (ESZKS Standards - Unified System of Protection against corrosion and aging of materials and products) [1, 2, 3, 4].
The purpose of the ESZKS is to ensure and maintain a given level of quality of products, structures and materials using means and methods of protection against corrosion, aging and biodamage, taking into account the requirements of safety, ecology, compatibility and interchangeability, as well as the competitiveness of products and structures on the world market.
In addition to the ESZKS standards, requirements for corrosion resistance are also established by standards for certain types of structures and their parts, depending on the current corrosion factors.
For underground structures (including foundations), the corrosion hazard criteria are [5]:
- corrosive aggressiveness of the environment (soils, groundwater and other waters) in relation to the metal of the structure (including biocorrosive aggressiveness of soils);
- dangerous effect of stray direct and alternating currents.
Based on these criteria, it follows that the rate of metal corrosion in the soil depends on:
- Soil pH
. The lower the pH (acidic environment), the higher the corrosion rate. - electrical resistance of the soil
. The higher the soil resistance, the slower the corrosion rate.
It is also necessary to take into account the presence of an anti-corrosion coating that prevents corrosion.
Studies to determine the electrical resistance of the soil, taking into account possible increases in humidity and temperature changes, were carried out by the American Federal Highway Administration (FHWA) and are reflected in the manual for transport engineers [6, Table N-1] (Table 1).
Representative values of soil resistivity
Electrical resistance of soils
Table 1
| Soil Type Soil type | Resistivity ρ(Ωm) Resistance, Ohm |
| Clay, saturated silt Clay | 100 |
| Sandy or silty clay Loam | 250 |
| Clayey sand or saturated sand | 500 |
| Sand Sand | 1500 |
| Gravel Clastic soil | 5000 |
| Dry sand, rock | >5000 |
Determination of soil pH is possible based on research by the American Iron and Steel Institute (AISI), according to which the pH value of soils ranges from 6.5 to 8.
Thus, knowing the indicators of electrical resistance and soil pH, it is possible to predict the service life of a metal screw pile for given soil conditions.
Approximate data on the corrosion rate of metals in various types of soils are also available in foreign design standards [7] (Table 2).
Corrosion rate of steel in soil
table 2
| Corrosion Rate, mm/yr (10-6 m/yr) Corrosion rate, mm/year (10-6 m/year) | Soil Type with or without groundwater (interpretation) Soil type (with or without groundwater) |
| 0.012 mm/yr (12∙10-6 m/yr) | Undisturbed natural soils(sand, silt, clay, schist) < shale clay, sediment, silt (sand, undisturbed structure> |
| 0.030 mm/yr (30∙10-6 μm/yr) | Undisturbed natural soils(sand, silt, clay, schist) Undisturbed soil (sand, silt, clay, shale) |
| 0.030 mm/yr (30∙10-6 μm/yr) | Aggressive natural soils (swamp, peat, marsh) Aggressive soils (peat, swamp) |
| 0.020 mm/yr (20∙10-6 μm/yr) | Non-compacted & non-aggressive fills (sand, silt, clay, schist) Sand, silt, clay, shale deposits |
| 0.050 mm/yr (50∙10-6 μm/yr) | Non-compacted aggressive fills (ashes, slags) Ash, slag |
As an example of determining the service life of a metal screw pile, consider a pile foundation installed under the floor structure of the Faceted Chamber of the Moscow Kremlin by the FUNDEKS company.
- The pile has a protective anti-corrosion coating based on epoxy compositions. The pile coating has increased resistance to soil corrosion. The service life of this coating according to the ESZKS is 50 years.
- Let us calculate steel under the influence of corrosion processes.
According to [7], the corrosion rate for technogenic soils is approx. 0.030 mm/year. Given a blade steel thickness of 5 mm, the corrosion period will be from 5/0.030 = 166.66 years.
Taking into account the timing of destruction of the protective coating, the estimated service life of the pile based on the corrosion rate of the blade is at least 200 years for this type of soil situation.
Bibliographic index
- GOST 9.101-2002 ESZKS. Basic provisions.
- GOST 9.102-91 ESZKS. Impact of biological factors on technical objects. Terms and Definitions.
- GOST 9.103-78 ESZKS. Temporary anti-corrosion protection of metals and products. Terms and Definitions.
- GOST 9.306-85 ESZKS. Metallic and non-metallic inorganic coatings. Notation.
- GOST 9.602-2005 “UNDERGROUND STRUCTURES General requirements for corrosion protection”
- Traffic Detector Handbook: Third Edition—Volume II. Publication no. FHWA-HRT-06-139
- EN 1993-5:2007. Eurocode 3. Design of steel structures. Part 5. Pile structures.
Educational and methodological manual “Protection of materials from corrosion”, page 21
MCC and corrosion cracking tests
These tests are carried out for austenitic, austenitic-ferritic and austenitic-martensitic stainless steels on samples in solutions of CuSO4×5H2O and H2SO4 with the addition of copper shavings and zinc dust. After boiling for a regulated time of 7 to 48 hours, the samples are bent to determine the network of cracks, which is a rejection characteristic. Determination of the penetration depth of MCC is carried out on a transverse section using a microscope.
The cracking test is carried out on testing machines or installations that make it possible to determine the level of safe stresses, the time of appearance of the first crack, and the nature of corrosion damage.
Corrosion indicators
Corrosion indicators generally characterize the degree of manifestation of various changes in materials as a result of corrosive influences.
Kinetics of electrochemical processes
The rate of corrosion can also be determined by the amount of dissolved metal, which according to Faraday's law is proportional to the amount of electricity.
Dm = Q×m E /F = i×S×t×m E /F = i×S×t×K,
where Q is the amount of electricity; Dm – weight reduction; F – Faraday number, K – electrochemical equivalent, K = m E / F; S – electrode area; m E – equivalent mass, m E = A/n, A – atomic mass, n – oxidation state.
Other indicators of corrosion
1. Depth corrosion index:
kn = P/t,
mm/year.
P is the average depth of corrosion damage per unit time.
2. Indicator of the rate of formation of a film of corrosion products:
kh = Dh/t,
mm/year
:
3. Mass change indicator:
km - , km + - change in the mass of the metal sample as a result of corrosion, per unit surface S of the metal per unit time: km ± = Dm/(S×t),
g/m 2 h,
Dm - increase or decrease in the mass of the metal sample during time t after removal of corrosion products.
The formula for converting km to depth indicator is: kn = km - ×8.76/r met
,
mm/year
.
4. Volumetric corrosion index
: kob = V(well)/(S×t),
cm 3 / cm 2 h
where V(well) = V×273×(P – PH2O)/(T×760); V is the volume of absorbed O2 during corrosion with oxygen depolarization or the volume of hydrogen released during corrosion with hydrogen depolarization.
5. Current corrosion indicator:
(
- corrosion current density) icor = Jcor/S, A/cm 2,
Jcor - corrosion current,
A
Formula for converting km - into current indicator: icor = km - ×/(k E ×1000), A/cm 2
k E is the electrochemical equivalent of the metal, k E = A/(n×F), A is the atomic mass of the metal,
g/mol,
n is the valency of the metal undergoing corrosion, F is the Faraday number, F = 26.8
A × h.
Formula for converting the volumetric corrosion index into the current icor
= kob/k E,
A/ cm 2
, k E is the electrochemical equivalent of the element, k E H2 = (22.4 × 10 3 /2)/26.8,
cm 3 / (A × h);
k E O2 =(22.4×10 3 /4)/26.8,
cm 3 /(A ×h).
6. Mechanical corrosion indicator
: ks = Dsb/sb×100% sb - the tensile strength of the metal before the onset of corrosion; Dsb is the change in the tensile strength of the metal during the corrosion process over time t.
7. Indicator of change in electrical resistance
: kR = DR/R×100%, R – electrical resistance of the sample before the onset of corrosion; DR is the change in the electrical resistance of a metal sample during the corrosion process over time t.
8. Focal corrosion indicator
: kО - the number of corrosion foci that appear on a unit of metal surface over a certain period of time of product operation.
9. Corrosion susceptibility index
: kС – service life (test) before the start of the corrosion process t in days. The beginning of the corrosion process is determined by the state of the metal surface, in which the corrosion damage has reached 1% of the area.
Corrosion
In terms of terms, corrosion is the destruction of metal caused by chemical and electrochemical processes developing on the surface of the metal during its interaction with the external environment. Other materials, such as concrete, building stone, wood, are also subject to destruction from corrosion; corrosion of polymers, in turn, is called destruction, but this topic requires a separate discussion. The most familiar type of corrosion is the rusting of iron. Erosion is the impact on metal of such environmental factors as rain, wind, dust, which is why metal arches, metal bridge structures, metal trusses, supports and other structures where the main load-bearing structures are made of metal must be protected comprehensively.
The standards by which metal manufacturers work, the basic terms regarding the metal itself and its alloys are regulated and spelled out in the international ISO . This is what corrosion means according to the ISO 8044 standard - “corrosion is a physico-chemical or chemical interaction between a metal (alloy) and the environment, leading to a deterioration in the functional properties of the metal (alloy), the environment or the technical system that includes them.”
To understand the mechanisms of metal corrosion and methods of preventing corrosion, it is necessary first of all to consider the environment in which the metal undergoes corrosion and “corrodes”; such an environment is called a corrosive or aggressive environment (see Appendix No. 1). In the case of metals, when speaking about their corrosion, we mean the undesirable process of interaction of the metal with the environment. The physicochemical essence of the changes that metal undergoes during corrosion is metal oxidation. Any corrosion process is multi-stage: Contact of the corrosive medium or its individual components with the metal surface is necessary. It is known that most metals (except Ag, Pt, Cu, Au) occur in nature in the ionic state: oxides, sulfides, carbonates, etc., usually called metal ores. The ionic state is more favorable; it is characterized by lower internal energy. This is noticeable in the production of metals from ores and their corrosion. The absorbed energy during the reduction of metal from compounds indicates that the free metal has higher energy than the metal compound. This leads to the fact that the metal in contact with a corrosive environment tends to move into an energetically favorable state with a smaller energy reserve. That is, we can say that the root cause of corrosion is the thermodynamic instability of a system consisting of metal and components of the surrounding (corrosive) environment. A measure of thermodynamic instability is the free energy released when the metal interacts with these components. But free energy in itself does not yet determine the rate of the corrosion process, i.e., the value most important for assessing the corrosion resistance of a metal. In some cases, adsorption or phase layers (films) that appear on the metal surface as a result of the onset of the corrosion process form such a dense and impenetrable barrier that corrosion stops or is greatly inhibited. Therefore, under operating conditions, a metal with a greater affinity for oxygen may turn out to be more stable, not less (for example, the free energy of oxide formation for Cr or Al is higher than for Fe, and they are often superior to Fe in resistance).
Below are photographs of the ISO 8501-1 standard, in accordance with which the degree of corrosion is assessed. An assessment of the initial condition of the surface is carried out in order to determine the possible and/or necessary degree of cleaning, as well as to select the optimal method of surface preparation.
The following items are subject to assessment: DEGREE OF CORROSION, PRESENCE OF DEFECTS, PRESENCE OF FAT AND OIL CONTAMINATIONS.
A. The surface of a steel that is largely covered with tightly adherent mill scale, but is virtually free of rust.
B. A steel surface that has begun to rust and from which mill scale is beginning to fall off.
C. A surface of steel from which mill scale has disappeared by rusting, or from which it can be removed, and which exhibits isolated corrosion damage when viewed normally.
D. The surface of a steel from which mill scale has been removed by rusting and on which general corrosion appears when viewed normally.
Appendix No. 1
Corrosion categories under atmospheric environmental conditions according to ISO 12944-2 and ISO 9223.
| Loss of weight and thickness of zinc coating per year of operation | Typical examples for temperate climates | |||
| degree | weight loss g/m² | reduction in thickness, µm | outside | inside |
| C1 minor | ≤ 0,7 | ≤ 0,1 | – | Heated buildings with a neutral atmosphere, for example: offices, shops, schools, hotels. |
| C2 weak | > 0,7-5 | > 0,1-0,7 | Atmosphere with little pollution. Mainly rural areas. | Unheated buildings where condensation occurs, for example: warehouses, gyms. |
| C3 moderate | > 5-15 | > 0,7-2,1 | The atmosphere of the city and industrial zones. Moderate sulfur dioxide pollution. | Industrial premises with high humidity and low air pollution, for example: food production, laundries, breweries, dairies. |
| C4 strong | > 15-30 | > 2,1-4,2 | Industrial areas and coasts with moderate salt concentrations. | Chemical structures, swimming pools, houses over the water. |
| C5-I very strong (industrial) | > 30-60 | > 4,2-8,4 | Industrial areas with high humidity and aggressive atmosphere. | Buildings or areas with almost constant condensation and heavy pollution. |
| C5-M very strong (sea) | > 30-60 | > 4,2-8,4 | Coastal areas with high salt concentrations. | Buildings or areas with almost constant condensation and heavy pollution. |
| Note: In coastal areas with warm, humid climates, mass loss or thickness reduction may exceed the limits of category C5-M. | ||||
Laboratory work No. 1. Corrosion of metals.
Goal of the work:
Study the effect of changing conditions on the occurrence of corrosion.
Corrosion of metals is the destruction of metallic materials due to their chemical or electrochemical interaction with the external environment. Example of electrochemical corrosion: corrosion of metals in the atmosphere during moisture condensation on a metal surface (about 80% of metal structures are operated in atmospheric conditions - transport, plant equipment, bridges, rails, etc.), corrosion of various pipelines in the ground, corrosion of metals in the sea and river water, etc.
- AltGTU 419
- AltSU 113
- AmPGU 296
- ASTU 267
- BITTU 794
- BSTU "Voenmekh" 1191
- BSMU 172
- BSTU 603
- BGU 155
- BSUIR 391
- BelGUT 4908
- BSEU 963
- BNTU 1070
- BTEU PC 689
- BrGU 179
- VNTU 120
- VGUES 426
- VlGU 645
- VmedA 611
- VolgSTU 235
- VNU named after Dalia 166
- VZFEI 245
- VyatGSHA 101
- VyatGSU 139
- VyatGU 559
- GGDSK 171
- GomGMK 501
- GGMU 1966
- GGTU named after. Sukhoi 4467
- GSU named after Skorina 1590
- GMA named after. Makarova 299
- DSPU 159
- DalGAU 279
- DVGGU 134
- DVGMU 408
- DVGTU 936
- DVGUPS 305
- FEFU 949
- DonSTU 498
- DITM MNTU 109
- IvGMA 488
- ISUTU 131
- IzhSTU 145
- KemGPPK 171
- KemSU 508
- KGMTU 270
- KirovAT 147
- KGKSEP 407
- KSTA im. Degtyareva 174
- KnAGTU 2910
- KrasGAU 345
- KrasSMU 629
- KSPU named after. Astafieva 133
- KSTU (SFU) 567
- KGTEI (SFU) 112
- PDA No. 2 177
- KubGTU 138
- KubSU 109
- KuzGPA 182
- KuzGTU 789
- MSTU im. Nosova 369
- MSEU named after. Sakharova 232
- MGEC 249
- MGPU 165
- MAI 144
- MADI 151
- MGIU 1179
- MGOU 121
- MGSU 331
- MSU 273
- MGUKI 101
- MGUPI 225
- MGUPS (MIIT) 637
- MSUTU 122
- MTUSI 179
- KhAI 656
- TPU 455
- NRU MEI 640
- NMSU "Mining" 1701
- KhPI 1534
- NTUU "KPI" 213
- NUK them. Makarova 543
- HB 1001
- NGAVT 362
- NSAU 411
- NGASU 817
- NSMU 665
- NGPU 214
- NSTU 4610
- NSU 1993
- NSUEU 499
- Research Institute 201
- OmSTU 302
- OmGUPS 230
- SPbPK No. 4 115
- PGUPS 2489
- PSPU named after Korolenko 296
- PNTU named after. Kondratyuka 120
- RANEPA 190
- ROAT MIIT 608
- RTA 245
- RGGMU 117
- RGPU named after. Herzen 123
- RGPPU 142
- RGSU 162
- "MATI" - RGTU 121
- RGUniG 260
- REU im. Plekhanova 123
- RSATU im. Solovyova 219
- RyazSMU 125
- RGRTU 666
- SamSTU 131
- SPbGASU 315
- ENGEKON 328
- SPbGIPSR 136
- SPbGLTU named after. Kirova 227
- SPbGMTU 143
- SPbGPMU 146
- SPbSPU 1599
- SPbGTI (TU) 293
- SPbGTURP 236
- SPbSU 578
- GUAP 524
- SPbGUNIPT 291
- SPbGUPTD 438
- SPbGUSE 226
- SPbSUT 194
- SPGUTD 151
- SPbGUEF 145
- SPbGETU "LETI" 379
- PIMash 247
- NRU ITMO 531
- SSTU named after Gagarina 114
- SakhSU 278
- SZTU 484
- SibAGS 249
- SibGAU 462
- SibGIU 1654
- SibGTU 946
- SGUPS 1473
- SibGUTI 2083
- SibUPK 377
- SFU 2424
- SNAU 567
- SSU 768
- TRTU 149
- TOGU 551
- TGEU 325
- TSU (Tomsk) 276
- TSPU 181
- Tula State University 553
- UkrGAZHT 234
- UlSTU 536
- UIPKPRO 123
- USPU 195
- USTU-UPI 758
- USPTU 570
- USTU 134
- KhGAEP 138
- KhGAFK 110
- KHNAGH 407
- KhNUVD 512
- KhNU named after Karazina 305
- KNURE 325
- KhNEU 495
- CPU 157
- ChitGU 220
- SUSU 309
Basic principles of atmospheric corrosion
Atmospheric corrosion is the most common type of metal corrosion that occurs in a humid air environment: approximately 80% of metal structures, buildings, structures, bridges, machines, etc. operated in atmospheric conditions. A distinctive feature of atmospheric corrosion is that it does not occur in the bulk of the electrolyte, but in thin films. In this case, the corrosion process proceeds according to the laws of electrochemical kinetics, but has its own specific features.
The main factors influencing the rate of atmospheric corrosion are:
- atmospheric humidity;
- chemical composition of the atmosphere;
- the duration of periods of wetting and drying of moisture films.
Rice. 1. Main corrosive hazardous impurities in the atmosphere
Let us consider in more detail the mechanism of influence of the above factors on the intensity of corrosion destruction.
Atmospheric humidity
The air always contains some amount of water vapor. Air humidity is usually described numerically by the following factors:
- absolute humidity (amount of steam), g/m3;
- water vapor pressure, atm (Pa);
- relative humidity, (φ), calculated as (P/Psat)·100%, where P is the pressure of water vapor in the air; Psac is the pressure of saturated water vapor at a given temperature.
According to air humidity indicators, atmospheric corrosion can be classified as follows:
- dry atmospheric corrosion;
- wet atmospheric corrosion;
- wet atmospheric corrosion.
Dry atmospheric corrosion
occurs in very thin films (up to 10 nm) at an air humidity of 30-50% and is characterized by surface oxidation of the metal by a chemical mechanism with the formation of the corresponding oxides (the phenomenon of “tarnishing” of the metal). As a rule, it does not lead to serious corrosion damage.
Wet atmospheric corrosion
usually begins at relative air humidity above 70%. At this humidity, called critical, capillary condensation of moisture occurs and water begins to exhibit the properties of an electrolyte. Capillary condensation can be stimulated by surface roughness, various irregularities, metal contamination with solid particles (dust), etc. The thickness of the moisture films formed on the metal ranges from 0.01 to 1 microns and under these conditions a very intense supply of oxygen occurs to the metal surface, which leads to an acceleration of the corrosion process compared to the volume of the electrolyte. The mechanism of the wet atmospheric corrosion process is shown in Fig. 2.
Rice. 2. Wet atmospheric corrosion
The critical humidity may decrease due to the presence of contaminants (natural or anthropogenic) on the metal surface, which attract moisture from the air. For example, particles of ammonium salts adsorbed on a steel surface significantly reduce the critical humidity from 70-80% to 50% (see Fig. 3). Also, the critical humidity is reduced by corrosion products formed on the metal surface (especially steel corrosion products), which ultimately leads to the acceleration of corrosion processes.
Rice. 3. The influence of contaminants on capillary condensation of moisture on the metal surface
Wet atmospheric corrosion occurs when the thickness of the moisture film on the metal surface is from 1 to 1000 microns. The corrosion process slows down somewhat compared to wet atmospheric corrosion due to the difficulty of oxygen diffusion to the metal surface and is more similar to ordinary electrochemical corrosion.
Chemical composition of the atmosphere
The corrosive aggressiveness of the atmosphere is determined not only by humidity, but also by various chemicals: solid and gaseous. The air contains various gases of biogenic, natural and anthropogenic origin (SO2, SO3, NO2, N2O3, N2O5, H2S, Cl2, etc.), as well as particles of solid substances (chlorides, sulfates, silicates, dust particles, etc.) (Fig. . 1). All these substances can dissolve in films of condensed moisture, increasing its corrosiveness. The most corrosive gases are SO2 and SO3, which form aerosols of sulfurous and sulfuric acids with water vapor in the atmosphere. In general, the classification of corrosive aggressiveness of atmospheric media is given in the following standards:
- ISO 9223: “Corrosion of metals and alloys. Corrosive activity of the atmosphere. Classification";
- ISO 12944: “Varnishes and paints. Protection of steel structures from corrosion using protective paint systems.”
Duration of periods of wetting and drying of moisture films
Structures in an open atmosphere are exposed to precipitation, aggressive gases, aerosols and other factors. The total duration of presence of a film of moisture on a metal surface is determined as the total duration of rain and dew, exposure to fog and thaws in winter, as well as the duration of drying of the surface. When the moisture film thickness is more than 30 microns (wet corrosion), the rate of the corrosion process is determined by the rate of moisture evaporation, time and frequency of rewetting. The rate of evaporation depends, in turn, on relative humidity φ, temperature, and air exchange rate.
It should be noted that metal wetting processes can reduce the overall rate of atmospheric metal corrosion by washing away corrosive adsorption films and cleaning the metal surface from dust and solid salts.
Do you want to learn more about corrosion of metal structures and anti-corrosion protection methods?
Download our specialized training and reference application “Corrosion Protection”
Metal corrosion rate. Methods for assessing corrosion processes
Corrosion rate is a multifactorial parameter that depends on both external environmental conditions and the internal properties of the material. In the regulatory and technical documentation there are certain restrictions on the permissible values of metal destruction during the operation of equipment and building structures to ensure their trouble-free operation. In design, there is no universal method for determining corrosion rate. This is due to the complexity of taking into account all factors. The most reliable method is to study the operating history of the facility.
Criteria
You will be interested in: Isobaric, isochoric, isothermal and adiabatic processes for an ideal gas
Currently, several corrosion rate indicators are used in equipment design:
- According to the direct method of assessment: reduction in the mass of a metal part per unit surface - weight indicator (measured in grams per 1 m2 per 1 hour); depth of damage (or permeability of the corrosion process), mm/year; the amount of released gas phase of corrosion products; the length of time during which the first corrosion damage appears; the number of corrosion centers per unit surface area that appeared over a certain period of time.
- According to indirect assessment: current strength of electrochemical corrosion; electrical resistance; change in physical and mechanical characteristics.
The first indicator using the direct assessment method is the most common.
Calculation formulas
In the general case, weight losses that determine the rate of metal corrosion are found using the following formula:
where q is the reduction in metal mass, g;
S – surface area from which the material was transferred, m2;
t – time period, hours.
For sheet metal and shells made from it, the depth indicator is determined (mm/year):
m is the depth of penetration of corrosion into the metal.
There is the following relationship between the first and second indicators described above:
where ρ is the density of the material.
Main factors influencing the corrosion rate
The rate of metal destruction is influenced by the following groups of factors:
- internal, related to the physical and chemical nature of the material (phase structure, chemical composition, surface roughness of the part, residual and operating stresses in the material, and others);
- external (environmental conditions, speed of movement of a corrosive environment, temperature, atmospheric composition, presence of inhibitors or stimulants, and others);
- mechanical (development of corrosion cracks, destruction of metal under the influence of cyclic loads, cavitation and fretting corrosion);
- design features (choice of metal grade, presence of gaps between parts, roughness requirements).
2.Classification of corrosion
2.1.Types of corrosion. Classification by type, geometric nature of corrosion damage on the surface or in the volume of the metal.
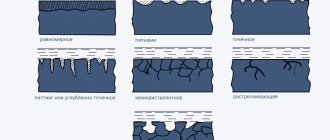
Corrosion that has affected the entire surface of the metal is called continuous. Continuous corrosion is divided into uniform and uneven, depending on the depth of corrosion destruction in different areas. With local corrosion, the lesions are local and leave a significant (sometimes overwhelming) part of the surface practically unaffected. Depending on the degree of localization, corrosion spots, ulcers and spots are distinguished. Pitting may begin as surface corrosion extending laterally under a very thin (e.g. riveted) layer of metal, which then blisters or peels off. The most dangerous types of local corrosion are intercrystalline (intercrystalline), which, without destroying the grains of the metal, moves deeper along their less resistant boundaries, and transcrystalline, which cuts the metal with a crack directly through the grains. Leaving almost no visible marks on the surface, these lesions can lead to a complete loss of strength and destruction of the part or structure. Knife corrosion is close in nature to them, as if cutting the metal along the weld with a knife when using some alloys in particularly aggressive solutions. Sometimes a special distinction is made between surface filamentous corrosion, which develops, for example, under non-metallic coatings, and layer-by-layer corrosion, which occurs predominantly in the direction of plastic deformation. Selective corrosion is specific, in which even individual components of solid solutions can be selectively dissolved in the alloy (for example, dezincification of brass).
2.2 Classification of corrosion according to the mechanism of reactions of interaction of metal with the environment (chemical and electrochemical corrosion).
Corrosion is chemical if, after breaking the metal bond, the metal atoms are directly connected by a chemical bond with those atoms or groups of atoms that are part of the oxidizing agents that take away the valence electrons of the metal. Chemical corrosion is possible in any corrosive environment, but most often it is observed in cases where the corrosive environment is not an electrolyte (gas corrosion, corrosion in non-conductive organic liquids). The rate of chemical corrosion is most often determined by the diffusion of metal particles and oxidizer through the surface film of corrosion products (high-temperature oxidation of most metals by gases), sometimes by the dissolution or evaporation of this film (high-temperature oxidation of W or Mo), its cracking (oxidation of Nb at high temperatures) and occasionally – convective delivery of the oxidizing agent from the external environment (at very low concentrations). Corrosion is electrochemical if, upon leaving the metal lattice, the resulting cation enters into contact not with the oxidizing agent, but with other components of the corrosive environment; electrons are transferred to the oxidizing agent, which are released during the formation of the cation. Such a process is possible in cases where there are two types of reagents in the environment, of which some (solvating or complexing) are capable of combining with stable bonds with a metal cation without the participation of its valence electrons, while others (oxidizing agents) can attach valence electrons of the metal without retaining cations around you. Solutions or melts of electrolytes, where solvated cations retain significant mobility, have similar properties. Thus, during electrochemical corrosion, the removal of an atom from a metal lattice (which is the essence of any corrosion process) is carried out as a result of two independent, but coupled, interconnected electrochemical processes: anodic - the transition of solvated metal cations into solution, and cathodic - binding oxidizer of released electrons. It follows that the process of electrochemical corrosion can be slowed down not only by directly inhibiting the anodic process, but also by influencing the speed of the cathodic one. The two most common cathodic processes are the discharge of hydrogen ions (2e + 2H+ = H2) and the reduction of dissolved oxygen (4e + O2 + 4H+ = 2H2O or 4e + O2 + 2H2O = 4OH-), which are often called hydrogen and oxygen depolarization, respectively. The anodic and cathodic processes, with one probability or another and in one order or another, occur at any points on the metal surface where cations and electrons can interact with the components of the corrosive environment. If the surface is homogeneous, then cathodic and anodic processes are equally probable over its entire area; in such an ideal case, corrosion is called homogeneous-electrochemical (thus noting the absence of any heterogeneity in the probability distribution of electrochemical processes at any point on the surface, which, of course, does not exclude the thermodynamic heterogeneity of the interacting phases). In reality, there are areas on metal surfaces with different conditions for the delivery of reacting components, with different energy states of atoms or with different impurities. In such areas, either anodic or cathodic processes may occur more vigorously, and corrosion becomes heterogeneous-electrochemical.
2.3. Classification of corrosion by type of corrosive environment
Some corrosive environments and the destruction they cause are so characteristic that the corrosion processes occurring in them are also classified by the name of these environments. As a rule, metal products and structures are exposed to many types of corrosion - in these cases they speak of the action of so-called mixed corrosion. Gas corrosion – corrosion in a gas environment at high temperatures. Atmospheric corrosion – corrosion of a metal under atmospheric conditions with humidity sufficient to form an electrolyte film on the metal surface (especially in the presence of aggressive gases or aerosols of acids, salts, etc.). A feature of atmospheric corrosion is the strong dependence of its speed and mechanism on the thickness of the moisture layer on the metal surface or the degree of moisture of the resulting corrosion products. Liquid corrosion is corrosion in liquid media. According to the conditions of exposure of the liquid medium to the metal, this type of corrosion is also characterized as corrosion under complete immersion, under partial immersion, under variable immersion, which have their own characteristic features. Underground corrosion is corrosion of metal in soils. A characteristic feature of underground corrosion is the large difference in the rate of oxygen delivery (the main depolarizer) to the surface of underground structures in different soils (tens of thousands of times).
2.4. Classification of corrosion according to the nature of additional effects
Stress corrosion develops in the area of tensile or bending mechanical loads, as well as residual deformations or thermal stresses. Stress corrosion usually leads to transcrystalline corrosion cracking, which is subject to, for example, steel cables and springs in atmospheric conditions, carbon and stainless steels in steam power plants, high-strength titanium alloys in sea water, etc. Under alternating loads, corrosion can occur fatigue, expressed in a more or less sharp decrease in the fatigue limit of the metal in the presence of a corrosive environment. Corrosion erosion (or friction corrosion) is the accelerated wear of metal under the simultaneous influence of mutually reinforcing corrosive and abrasive factors (sliding friction, flow of abrasive particles, etc.). Related to it, cavitation corrosion occurs during cavitation regimes of flow of an aggressive medium around a metal, when the continuous emergence and “collapse” of small vacuum bubbles creates a stream of destructive microhydraulic shocks affecting the metal surface. A close variety can be considered fretting corrosion, which is observed at the points of contact between tightly compressed or rolling parts, if microscopic shear displacements occur as a result of vibrations between their surfaces. Leakage of electric current through the boundary of a metal with an aggressive environment causes, depending on the nature and direction of the leakage, additional anodic and cathodic reactions that can directly or indirectly lead to accelerated local or general destruction of the metal (stray current corrosion). Similar destruction, localized near the contact, can be caused by contact in the electrolyte of two dissimilar metals forming a closed galvanic cell - contact corrosion. In narrow gaps between parts, as well as under a loose coating or build-up, where the electrolyte penetrates, but the access of oxygen necessary for passivation of the metal is difficult, crevice corrosion can develop, in which the dissolution of the metal mainly occurs in the crack, and cathodic reactions partially or completely occur next to her on an open surface. It is also customary to distinguish between biological corrosion, which occurs under the influence of waste products of bacteria and other organisms, and radiation corrosion, which occurs under the influence of radioactive radiation.
Physicochemical characteristics
You may be interested in: Such ordinary people, or the meaning of “why not”
The most important among internal corrosion factors are the following:
- Thermodynamic stability. To determine it in aqueous solutions, Pourbaix reference diagrams are used, the abscissa of which is the pH of the medium, and the ordinate is the redox potential. A positive shift in potential means greater stability of the material. It is roughly defined as the normal equilibrium potential of the metal. In reality, materials corrode at different rates.
- Atom position in the periodic table of chemical elements. The metals most susceptible to corrosion are alkali and alkaline earth metals. The rate of corrosion decreases as the atomic number increases.
- Crystal structure. It has an ambiguous effect on destruction. The coarse-grained structure itself does not lead to an increase in corrosion, but is favorable for the development of intercrystalline selective destruction of grain boundaries. Metals and alloys with a uniform phase distribution corrode uniformly, while those with a non-uniform phase distribution corrode according to a focal mechanism. The mutual arrangement of the phases performs the function of anode and cathode in an aggressive environment.
- Energy heterogeneity of atoms in a crystal lattice. Atoms with the highest energy are located in the corners of the edges of microroughness and are active centers of dissolution during chemical corrosion. Therefore, careful mechanical processing of metal parts (grinding, polishing, finishing) increases corrosion resistance. This effect is also explained by the formation of denser and more continuous oxide films on smooth surfaces.
Influence of acidity of the environment
You might be interested in: Comic nominations for teachers for graduation.
In the process of chemical corrosion, the concentration of hydrogen ions affects the following points:
- solubility of corrosion products;
- formation of protective oxide films;
- metal destruction rate.
At a pH in the range of 4-10 units (acidic solution), corrosion of iron depends on the intensity of oxygen penetration to the surface of the object. In alkaline solutions, the corrosion rate first decreases due to surface passivation, and then, at pH>13, increases as a result of the dissolution of the protective oxide film.
Each type of metal has its own dependence of the intensity of destruction on the acidity of the solution. Noble metals (Pt, Ag, Au) are resistant to corrosion in acidic environments. Zn and Al are quickly destroyed in both acids and alkalis. Ni and Cd are resistant to alkalis, but easily corrode in acids.
Hello student
THE CONCEPT OF CORROSION OF METALS
Corrosion is the destruction of metal under the chemical action of the environment.
Metals are found in nature mainly in the form of compounds with non-metallic elements, for example, iron - in the form of Fe2O3, etc., aluminum - Al2O3, copper - Cu2S, etc.
To obtain a metal in its pure form, energy is required, since metallurgical processes disrupt the equilibrium state between the metal and the substances with which it is associated in its natural state.
Metals obtained as a result of metallurgical processes, under the influence of atmospheric conditions (gases and moisture), again tend to transform into stable compounds with other substances, in other words, they corrode.
Currently, corrosion destroys about 1% of the metal in use every year.
In products and structures, as a result of corrosion, successive destruction of the surface can occur, the formation of shells and the acquisition of a spongy structure by the metal. The development of modern corrosion theory is based on the research of G.V. Akimov, N.A. Izgaryshev, N.D. Tomashev and others.
TYPES OF CORROSION
There are two main types of metal corrosion: chemical and electrochemical.
Chemical corrosion is the process of destruction of metal in dry gases or in liquids that do not conduct electrical current (oil, gasoline).
This kind of corrosion occurs, for example, during the oxidation of metal in thermal furnaces, during the corrosion of furnace bonds, etc. The degree of chemical corrosion increases greatly with increasing temperature; As an example, we can point to tarnish colors.
The rate of metal destruction during chemical corrosion is determined by the degree of affinity of the metal with oxygen and the properties of the film formed (its density, the absence of pores in it, the strength of attachment of the film to the metal).
In fig. 158 shows a graph of iron oxidation depending on temperature.
Experience shows that the oxide film exhibits protective properties if the volume of the oxide is greater than the volume of the oxidized metal; Thus, for Ca, Cu and Fe, the ratio of the volume of oxide to the volume of oxidized metal is, respectively, 0.78; 1.70 and 2.06. The protective properties of the film of copper and iron oxides can be seen in Fig. 159; The graph shows that the film does not exhibit protective properties for Ca.
Alternating heating and cooling of the metal increases the rate of corrosion, as temperature fluctuations disrupt the integrity of the film.
Chemical corrosion can destroy metal not only from the surface, but also between crystalline grains, penetrating deep into the metal. In the latter case, corrosion is called intergranular.
Electrochemical corrosion is the destruction of metal in contact with liquids that conduct electric current. The phenomena occurring here are similar to those that occur in a galvanic cell.
A metal plate immersed in a liquid that conducts current releases positively charged particles - ions, and is itself charged negatively - electrons. Electrostatic equilibrium is established between the plate and the liquid.
The number of ions released by different metals is different, therefore, their potentials are also different.
When connecting plates of dissimilar metals immersed in a liquid with a conductor located outside the liquid, a current (Fig. 160) of electrons from A to B will flow through the latter; At the same time, a current of ions from A to B arises in the liquid. On the surface of plate B, the ions are neutralized by electrons flowing through the conductor.
A galvanometer connected to the circuit will show the flow of electricity in the circuit.
As a result, plate A (anode) is destroyed.
In a galvanic couple, the metal that releases a greater number of positively charged ions is destroyed and, therefore, itself receives a higher negative charge. To compare the electropositive properties of metals, their electrode potentials are measured under the same conditions with respect to the potential of the hydrogen electrode, which is assumed to be zero.
As the positive charge in pair with the hydrogen electrode decreases or, as they say, as the electropositive properties decrease, the metals are arranged in a series called the voltage series: Au, Pt, Hg, Ag, Cu, Bi, Sb, (H2), Pb , Sn, Ni, Co, Cd, Fe, Cr, Zn, Mn, Al, Mg, Be, Na, K. Li.
This series was compiled on the condition that the determination of electrode potentials was carried out at a normal solution concentration (in a solution containing 1 g equivalent of ions of a given metal per 1 liter at 18 ° C).
Any metal occupying a more right-hand position in this row will be destroyed in contact with those occupying a more left-hand position, and the more strongly, the further these metals are from each other in the row.
Depending on the electrolyte, the order of arrangement of these metals in a number of voltages may be different, and therefore, the relative ability to corrode may also change.
The rate of corrosion is characterized by the strength of the current between them.
The magnitude of this current can decrease and corrosion slows down, due to the polarization of the electrodes and, first of all, the cathode, on which electrons accumulate during the corrosion process. Depolarization of the cathode (removal of electrons) can occur in two ways: a) through the release of hydrogen at the cathode with the absorption of electrons; this depolarization process takes place in an acidic environment; b) by removing electrons from the cathode with gaseous oxygen in solution with the formation of hydroxyl ions according to the equation
This type of depolarization is characteristic of neutral solutions.
Polarization reduces the electromotive force of the pair and can even change the sign of the potential. Therefore, the intensity of the process of metal destruction during electrochemical corrosion also depends on the degree of polarization of the electrodes: the greater the polarization, the weaker the corrosion and vice versa.
The following types of electrochemical corrosion are distinguished: galvanic corrosion and corrosion under the influence of stray currents.
Galvanic corrosion is the process of metal destruction when dissimilar metals come into contact in the presence of an electrolyte; As an example, we can point to the destruction of iron in steam boilers that form a galvanic couple with furnace copper. In many cases, the process of galvanic corrosion is caused by the heterogeneity of the structure of the metal or alloy. As an example, we can point to the destruction of perlite; here ferrite is negatively charged (relative to cementite), subject to destruction in the presence of moisture. Corrosion that occurs as a result of structural heterogeneity, i.e., due to the presence of trace elements, is called microcorrosion or structural corrosion.
The widespread opinion that only alloys are subject to microcorrosion cannot be considered completely correct, since heterogeneity of the structure can also occur in pure metal, for example, due to cold hardening. In addition, some possibility of microcorrosion of pure metal may be caused by potential dissimilarity at different points on the metal surface, which is never absolutely homogeneous.
However, it is obvious that pure metals can be subject to microcorrosion to a lesser extent compared to an alloy with a complex structure, and that microcorrosion is less pronounced the less heterogeneous the metal surface is.
The currents that cause microcorrosion are very small: their value can be approximately 10-6-10-7 A/cm2. The small magnitude of these currents is compensated by the duration of their action.
In areas served by electric railways, currents returning from the network to the station through the rails can partially branch off, passing through the metal of objects located in the soil (water pipes, frames of reinforced concrete structures, etc.). In this case, the soil containing salt solutions plays the role of an electrolyte; The metal that is the anode is destroyed. This type of corrosion is called stray current corrosion.
Factors affecting corrosion rate
Internal factors. Internal factors affecting the corrosion rate include chemical composition, structure, internal stresses and the condition of the metal surface. Pure metals, other things being equal, are less susceptible to corrosion than alloys.
Alloys with a solid solution structure are less susceptible to corrosion than others.
Internal stresses of the material contribute to its corrosion; hardened material corrodes more than material with a normal structure. The presence of internal stresses and hardening contribute to the formation of galvanic couples.
The smoother the surface of the metal, the less it corrodes; When the surface is damaged, the metal begins to corrode faster. The reason for this is the fact that a smooth surface is better covered with a protective film.
External factors. External factors influencing the rate of corrosion include the effect of the environment on the metal: water, acids, alkalis, salts and gases, as well as ambient temperature.
Metal corrosion fatigue
Corrosion fatigue of a metal is the destruction of a metal that is under the simultaneous influence of cyclic stresses and factors that cause corrosion.
The reason for the rapid destruction of the metal is, on the one hand, the destruction of the oxide film and, consequently, the failure to use its protective properties, and on the other hand, the surface destruction of the metal caused by corrosion, which contributes to the occurrence of fatigue cracks. Under the influence of corrosion, the fatigue limit of the metal is reduced in some cases by 65% compared to the normal value.
Download abstract: You do not have access to download files from our server. HOW TO DOWNLOAD HERE
Archive password: privetstudent.com
Composition and concentration of neutral solutions
The rate of corrosion in neutral solutions depends to a large extent on the properties of the salt and its concentration:
- The hydrolysis of salts in a corrosive environment produces ions that act as activators or retarders (inhibitors) of metal destruction.
- Those compounds that increase pH also increase the rate of the destructive process (for example, soda ash), and those that reduce acidity reduce it (ammonium chloride).
- In the presence of chlorides and sulfates in the solution, destruction is activated until a certain salt concentration is reached (which is explained by the intensification of the anodic process under the influence of chlorine and sulfur ions), and then gradually decreases due to a decrease in oxygen solubility.
Some types of salts are capable of forming a poorly soluble film (for example, iron phosphate). This helps protect the metal from further destruction. This property is used when using rust neutralizers.
Corrosion inhibitors
Corrosion retarders (or inhibitors) differ in their mechanism of action on the redox process:
- Anode. Thanks to them, a passive film is formed. This group includes compounds based on chromates and dichromates, nitrates and nitrites. The last type of inhibitors is used for inter-operational protection of parts. When using anodic corrosion inhibitors, it is necessary to first determine their minimum protective concentration, since addition in small quantities can lead to an increase in the rate of destruction.
- Cathode. The mechanism of their action is based on reducing the oxygen concentration and, accordingly, slowing down the cathodic process.
- Shielding. These inhibitors isolate the metal surface by forming insoluble compounds that are deposited as a protective layer.
The last group includes rust neutralizers, which are also used to remove oxides. They usually contain orthophosphoric acid. Under its influence, phosphating of the metal occurs - the formation of a durable protective layer of insoluble phosphates. Neutralizers are applied with a spray or roller. After 25-30 minutes the surface becomes white-gray. After the composition has dried, paint and varnish materials are applied.
Methods to reduce corrosion: mechanism and effectiveness
The ability of a painted surface to resist corrosion processes depends on which corrosion mechanism predominates. For example, with constant exposure to a chemically active medium over time, the potential difference between the outer surface of a metal product and its internal volumes changes significantly. In this case, corrosive currents arise, intensifying the corrosion process (a phenomenon that often causes the destruction of steel pipes in underground pipelines). Here, painting does not have any effect, since the chemical composition of the surface covered with a layer of paint does not change over time.
Metal coating
It’s a different matter when the surface is covered with a metal that has a negative electrolytic potential with respect to redox processes. When oxidation reactions predominate, it is more effective to protect steel by applying a surface coating containing aluminum and zinc - metals that, in terms of their oxygen activity, are “to the left” of iron.
Such processes - galvanizing and aluminizing - are widely used in the practice of anti-corrosion protection of steel components and individual parts located in an oxidizing environment. Painting in these situations is of an auxiliary nature, to increase the decorative characteristics of the surface.
In a reducing environment, the process of formation of iron hydrides can be effectively blocked by creating surface coatings of metals located “to the right” of hydrogen: these are copper and all noble metals. Copper plating, although used in practice, is usually performed for relatively small surfaces, since it is a very costly process in terms of finances. It is for such situations that coloring can and should be used.
Coloring
The protective role of paints is that they always contain corrosion inhibitors - components that slow down the rate of scaling processes over time. The chemical formulas of inhibitor substances are designed in such a way that, as a result, the appearance of rust is stopped. The elasticity of modern coloring compositions allows coatings to successfully withstand surface stresses, which provoke the onset of corrosion processes.
The anti-corrosion properties of paints increase if they contain organosilicon polymers, which increase the ability of the painted surface to withstand changes in humidity and temperature, regardless of the time of year. However, such paints have two significant disadvantages:
- poisonous;
- are ineffective under conditions of the electrolytic corrosion mechanism.
Thus, properly selected coloring compositions can effectively block corrosion processes. To do this, they must contain corrosion inhibitors, have sufficient elasticity and mechanical strength that changes slightly over time.
Mechanical impact
Increased corrosion in an aggressive environment is facilitated by such types of mechanical impact as:
- Internal (during molding or heat treatment) and external (under the influence of externally applied load) stresses. As a result, electrochemical heterogeneity occurs, the thermodynamic stability of the material decreases, and corrosion cracking occurs. Destruction occurs especially quickly under tensile loads (cracks form in perpendicular planes) in the presence of oxidizing anions, for example, NaCl. A typical example of devices susceptible to this type of destruction are parts of steam boilers.
- Alternating dynamic effects, vibrations (corrosion fatigue). There is an intensive decrease in the fatigue limit, multiple microcracks are formed, which then merge into one large one. The number of cycles before failure largely depends on the chemical and phase composition of metals and alloys. Pump axles, springs, turbine blades and other equipment elements are susceptible to such corrosion.
- Friction of parts. Rapid corrosion is caused by mechanical wear of protective films on the surface of the part and chemical interaction with the environment. In liquid, the rate of destruction is lower than in air.
- Cavitation impact. Cavitation occurs when the continuity of fluid flow is disrupted as a result of the formation of vacuum bubbles, which collapse and create a pulsating effect. As a result, deep local damage occurs. This type of corrosion is often observed in chemical apparatus.
Practice of corrosion testing of metals
Indicators of corrosion are climatic factors - temperature, composition and relative humidity of the environment, the nature of the distribution of external loads. It is also necessary to take into account changes in illumination by time of day, amount of precipitation, and possible air pollution. For example, in areas of smoke waste emissions near chemical plants and metallurgical plants, accompanied by a sharp increase in the percentage of SO2, corrosion processes are sharply intensified.
Quantitative dependences of corrosion on time can be used as indicators of corrosion activity:
- Linear - most often this is typical for metal surfaces that do not have a protective coating.
- Exponentially decreasing - found during acid corrosion of ordinary metals and alloys.
- Exponentially increasing - when there is a protective coating on the surface of the part.
The intensity of rust formation under such conditions is reduced by:
- low wind speed;
- reduced cyclicity in time of changes in relative humidity indicators;
- the nature of the impact of a corrosive environment on the surface.
With little or no wind, there are no conditions for mixing the flow washing the contact surface of the steel. During long phases of low and high humidity throughout the year, a film of surface rust has time to form, swell and separate from the base metal. The thickness of the surface will decrease, but the corrosion processes are forced to “start” all over again, and this requires not only time, but also suitable conditions - wind or changes in the chemical composition of the air, which is not always the case.
Moisture, acid or alkali can reach the surface of the steel in the form of drops or streams. The first method is typical for areas with high precipitation, and the second is for the unfavorable environment in which the part or metal structure operates.
Design factors
You may be interested in: Dig or drip?
How to write correctly? When designing elements operating in aggressive conditions, it must be taken into account that the corrosion rate increases in the following cases:
- upon contact of dissimilar metals (the greater the difference in electrode potential between them, the higher the current strength of the electrochemical destruction process);
- in the presence of mechanical stress concentrators (grooves, grooves, holes, etc.);
- with low cleanliness of the treated surface, since in this case local short-circuited galvanic pairs arise;
- when there is a significant difference in the temperature of individual parts of the apparatus (thermogalvanic elements are formed);
- in the presence of stagnant zones (cracks, gaps);
- during the formation of residual stresses, especially in welded joints (to eliminate them, it is necessary to provide heat treatment - annealing).
Assessment methods
There are several ways to assess the rate of destruction of metals in aggressive environments:
- Laboratory - testing samples in artificially simulated conditions close to real ones. Their advantage is that they can reduce research time.
- Field – carried out in natural conditions. They take a long time. The advantage of this method is to obtain information about the properties of the metal under further operating conditions.
- Full-scale – testing of finished metal objects in a natural environment.
Source